Aging, Health, and Longevity FAQ’s
Why do we age?
Aging is a biological process that results in progressive functional decline and increased vulnerability to disease. Death due to aging is considered to be “natural”. However, aging does not have to be an inevitable consequence of the passage of time. In general terms, aging can be thought of as biological damage that occurs at a rate that outstrips the body’s ability to repair it. There are many theories as to exactly why we age, although none have been definitively proven. However, it is unlikely that any single mechanism is wholly responsible for the aging process.
Is aging a disease?
Whether or not aging is a disease is a matter of debate. Aging does not directly cause death, but causes a wide range of age-related diseases and disorders that are eventually fatal. Aging could be considered to be the universal disease that causes all diseases of aging. Different age-related diseases could be considered to be the different manifestations of aging throughout the body, similar to how different cancers are manifestations of the same disease in different tissues. However, many argue that aging is too broad a concept to be called a disease, as it cannot yet be reduced to a few, specific processes.
How can we measure biological age?
Scientists estimate the true biological age of an organism (as opposed to their chronological age) by measuring biomarkers of aging. A biomarker of aging is a biological marker that changes in a predictable way as an organism ages, and can therefore be measured to assess the extent of aging in an organism’s tissues. Measuring biological age is challenging because there are many biomarkers of aging that could be taken into account, and said biomarkers may not change at the same rate from one organ to the next. This means that a single organism could effectively have multiple biological ages throughout their tissues. Some of the main mechanisms used as biomarkers of aging involve modifications to the DNA, such as DNA methylation, histone modification and telomere shortening. Others include levels of background inflammation, mitochondrial function, cellular senescence, levels of various proteins and signalling molecules, and many others.
What are hallmarks of aging?
Hallmarks of aging are the ‘common denominators’ of the aging process: a set of biological changes that are observed to correlate with the progression of aging and age-related diseases across a range different tissues and organisms. If a hallmark is commonly detected in age-related diseases, and genetic mutations affecting said hallmark can affect the progression of aging, we may suppose that it is fundamental to the ageing process in some way, and that interventions that target it might have the potential to slow aging.
9 hallmarks of aging are widely acknowledged: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication.
 The hallmarks of ageing.
The hallmarks of ageing.
Source
What is genomic instability?
A genome is the entire set of DNA in a cell. Genomic instability is a hallmark of aging, and refers to a high frequency of mutations within the genetic code. Such mutations impair a cell’s ability to function properly, make it more likely to become cancerous, and can cause the cell to stop dividing (senescence) or to self-destruct (apoptosis).
Genomic instability is usually kept to a minimum by highly efficient DNA repair mechanisms. However, as we age, we are exposed to more sources of DNA damage, and our repair mechanisms become less effective. Mutations that slip through the net are passed on to daughter cells during cell division, accumulating over time. The resulting loss of cells and of cell function can impair tissue and organ function and make us more susceptible to cancer. This article provides a more detailed explanation of genomic instability.
 DNA is constantly exposed to sources of damage, both from within the body and from outside. Failure to repair this damage leads to mutations which are then passed on to daughter cells.
DNA is constantly exposed to sources of damage, both from within the body and from outside. Failure to repair this damage leads to mutations which are then passed on to daughter cells.
Source
What is telomere attrition?
Telomere attrition or telomere shortening is a hallmark of aging, and refers to the shortening of the telomeres each time the DNA is replicated during cell division. Telomeres are sequences of repetitive DNA that contain no useful genetic information, and can be likened to the plastic tips on shoelaces, protecting the ends of the chromosomes from damage.
After a enough cell divisions (40-60 in humans) telomeres will become too short to adequately protect the genetic code. At this point, cells will usually either stop dividing (this is called replicative senescence) or ‘commit suicide’ (apoptosis). This is essential to protect against damage to the genetic code and the development of cancer. However, telomere attrition contributes to tissue dysfunction in old age as cells are depleted through senescence or apoptosis. A more detailed explanation of telomere attrition can be found in this article.
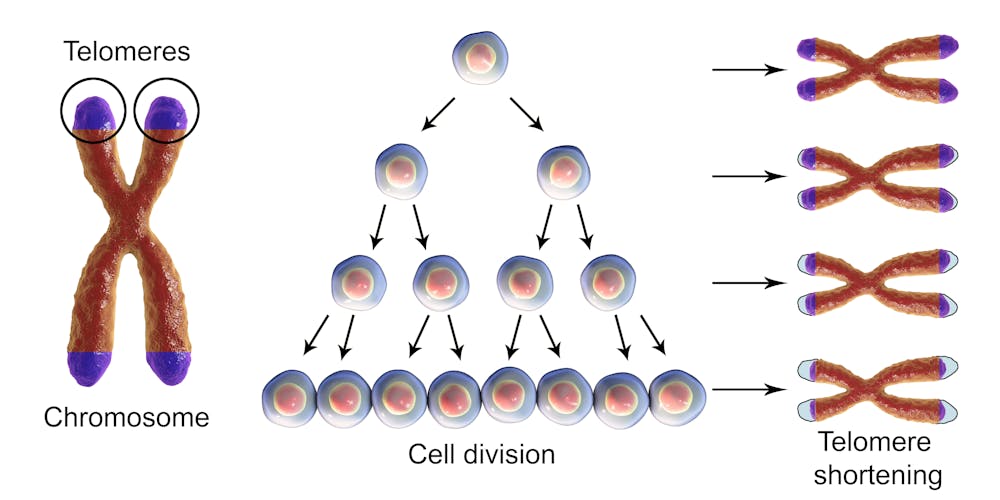 Telomere shortening during cell division.
Telomere shortening during cell division.
Source
What are epigenetic alterations?
From the Greek Epi, meaning ‘near’ or ‘upon’, the epigenome is the information that your cells have in addition to your DNA code, consisting of chemical compounds that do not change the DNA sequence but still affect gene activity. Epigenetic alterations are a hallmark of aging, and refer to changes to the epigenome that occur during aging as a result of damage to the DNA. Various lifestyle factors, such as diet and physical activity, can influence epigenetic alterations.
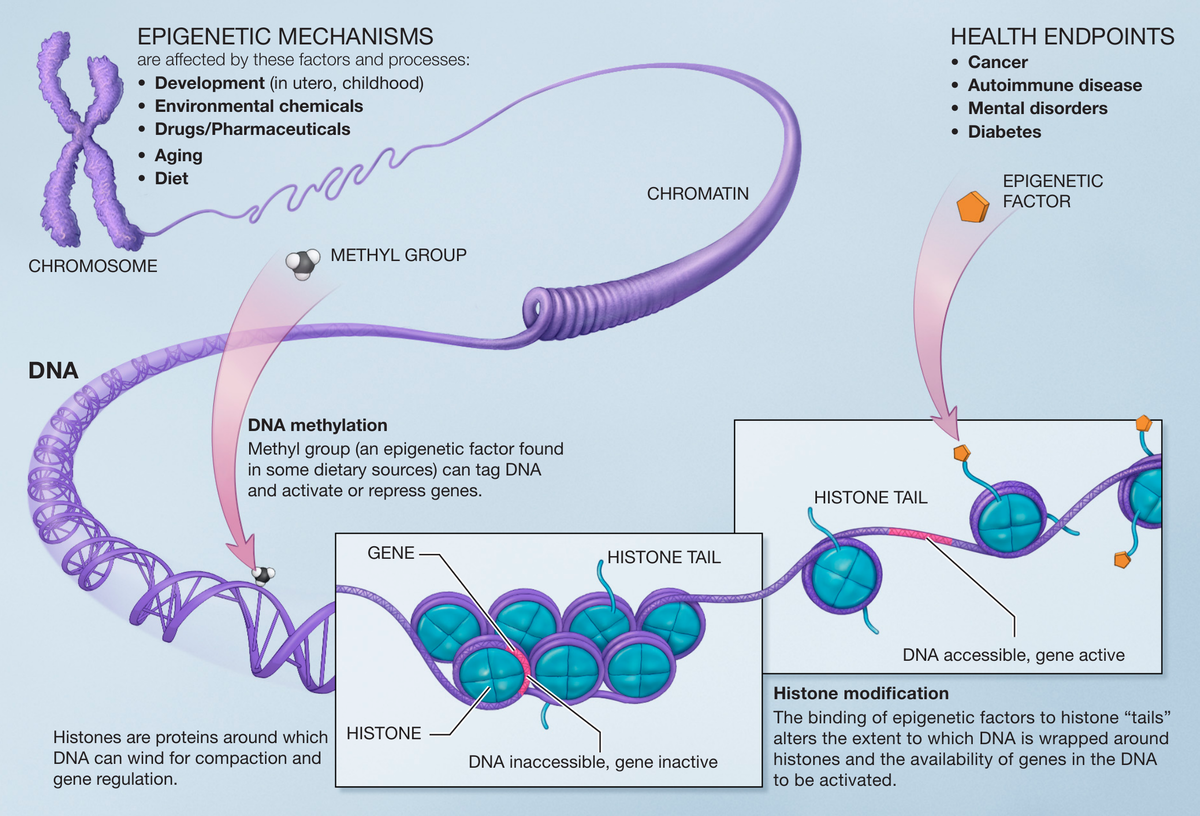 Depiction of DNA methylation and histone modification, two forms of epigenetic alteration.
Depiction of DNA methylation and histone modification, two forms of epigenetic alteration.
Source
Epigenetic alterations can change the activity of important genes, causing cells to function poorly or to die. Epigenetic alterations contribute to various age-related problems such as increased inflammation and impaired energy production by the mitochondria. This article provides a more detailed description of epigenetic alterations.
What is loss of proteostasis?
Proteostasis is essentially the overall ‘management’ of proteins by the cell, including building them correctly when they are needed, their continued maintenance, shuttling them around the cell and recycling/destroying them once they’ve become too damaged to do their job properly. Loss of proteostasis is a hallmark of aging, and refers to the decline in ability of cells to properly manage their proteins. In particular, cells become less able to repair dispose of damaged and potentially harmful proteins, such as those that have unfolded. The accumulation of such proteins is associated with many age-related diseases and processes, most famously neurodegenerative diseases like Alzheimer’s. A more detailed explanation of proteostasis can be found in this article.
 The potential fates of an unfolded protein: misfolding and aggregation; refolding by chaperone proteins; degradation by proteosome; degradation by lysosome.
The potential fates of an unfolded protein: misfolding and aggregation; refolding by chaperone proteins; degradation by proteosome; degradation by lysosome.
Source
What is deregulated nutrient sensing?
Deregulated nutrient sensing is a hallmark of aging, and describes how our cells fail to sense and respond appropriately to the levels of available nutrients. Changes in nutrient sensing pathways affect how cells distribute their energy between energy-saving and energy-demanding processes like cell division. Getting this balance wrong may accelerate age-related changes within the cell.
The most prominent aspect of deregulated nutrient sensing that occurs with age is insulin resistance, in which cells’ ability to control blood sugar in response to insulin is impaired. Insulin resistance is mostly associated with diabetes, but in truth insulin resistance promotes most major chronic diseases of aging including heart disease and cancer. This article provides a more detailed explanation of altered nutrient sensing.
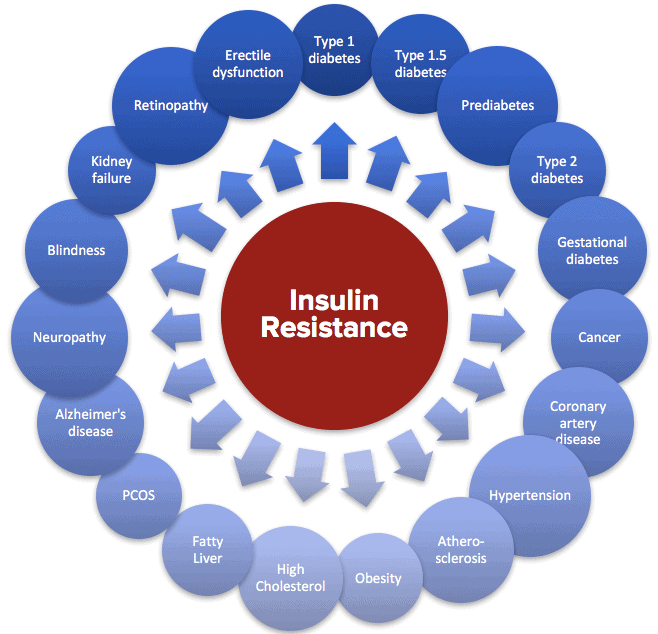 Insulin resistance drives or is implicated in a diverse range of diseases.
Insulin resistance drives or is implicated in a diverse range of diseases.
Source
What Is mitochondrial dysfunction?
Mitochondria are the power plants that produce the energy (ATP) needed for our cells to function. Mitochondrial dysfunction is a hallmark of aging, and refers to a decline in the mitochondria’s ability to function, including their ability to produce energy. This happens because mitochondria accumulate damage in their genetic code, some of which is kept separate from the rest of the human genome and therefore is not as well protected.
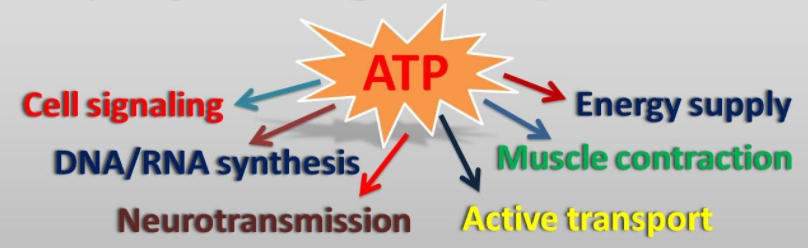 ATP, the cell’s ‘fuel’, is required for many vital processes.
ATP, the cell’s ‘fuel’, is required for many vital processes.
Source
Mitochondrial dysfunction means that there is less energy available for the cell, which may contribute to loss of function with age, particularly in tissues that require a lot of energy like muscle. In addition to producing energy, mitochondria are also important in a wide range of processes that are relevant to aging such as apoptosis (cell suicide), autophagy (the disposal of damaged cell components) inflammation and glucose metabolism. This article provides a more in-depth explanation of mitochondrial dysfunction.
What is cellular senescence?
Cellular senescence is a state in which cells no longer divide, and is one of the hallmarks of aging. Senescence occurs after a cell has divided a certain number of times (40-60 divisions in humans) and can also be induced when the cell sustains irreparable damage. Senescence is important in protecting against cancer, but becomes a problem in older age. This article provides a more detailed description of cellular senescence.
Cellular senescence can contribute to the depletion of stem cells, which are needed to maintain and repair tissues. Senescent cells are usually removed by the immune system, but immune cells are unable to clear senescent cells quickly enough in older age. Accumulating senescent cells release inflammatory molecules, growth factors and enzymes that digest extracellular proteins, which can result in the disruption of tissue structure and function while promoting cancer and inflammation.
 Telomere shortening and various sources of damage can induce senescence. This is beneficial so long as these cells are removed (blue), but detrimental if they are allowed to accumulate (red).
Telomere shortening and various sources of damage can induce senescence. This is beneficial so long as these cells are removed (blue), but detrimental if they are allowed to accumulate (red).
Source
What is stem cell exhaustion?
Stem cells are small populations of self-renewing cells which retain the ability to give rise to a limited set of cell types. They support our tissues and organs by dividing to generate new cells as needed. Stem cell exhaustion is a hallmark of aging, and refers to a decline in stem cell numbers and renewal capacity. Without stable populations of proliferating stem cells, tissues and organs lose their ability to recover from damage and begin to fail. This article contains a more detailed description of stem cell exhaustion.
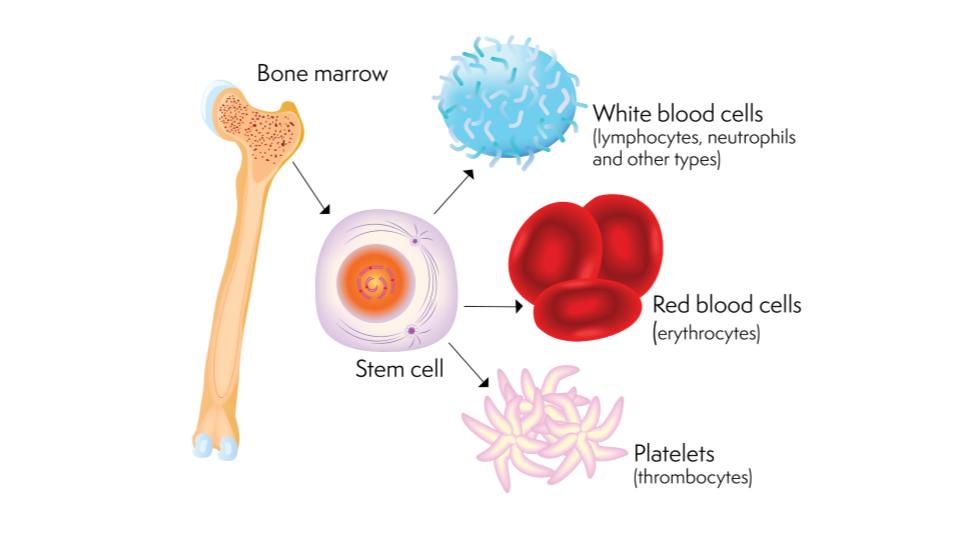 Multipotent stem cells in the bone marrow give rise to a limited selection of cell types.
Multipotent stem cells in the bone marrow give rise to a limited selection of cell types.
Source
Stem cell exhaustion with age results in a clear decline in regenerative capacity, meaning slower recovery from injury and illness. Stem cell exhaustion also impairs the function of the immune system, because there is a reduced ability to generate the necessary types of white blood cells to effectively combat pathogens.
What is altered intercellular communication?
Altered intercellular communication is a hallmark of ageing, and refers to changes in the signals that cells throughout the body send to eachother. Intercellular communication is like a biological telecoms network, allowing even distant cells to coordinate their activities. With age, an increasing number of harmful signals are released by certain cells (such as senescent cells), which can drive the ageing process in multiple organ systems. There may also be certain beneficial signals that help the body maintain a youthful state, but which decline with age. This article provides a more detailed explanation of how intercellular communication is disrupted in aging.
The most prominent aspect of altered intercellular communication is an increase in chronic background inflammatory signals known as ‘inflammaging’. These signals come from a variety of sources including senescent cells and in response to various sources of damage. Inflammation drives almost all chronic diseases of aging.
 How inflammation promotes disease in different organ systems.
How inflammation promotes disease in different organ systems.
Source
What is disabled macroautophagy?
Macroautophagy is a process that helps cells get rid of unwanted or damaged material, such as proteins, whole microbes and damaged organelles (cellular ‘organs’). This helps the cell stay healthy and function well. As we get older, macroautophagy becomes less effective and the cell accumulates more waste. This can affect how the cell works and cause problems for our health. Scientists think that macroautophagy is important for aging and they are studying how it works and how to improve it.
What is chronic inflammation?
Inflammation is a normal response of the body to fight infections or injuries, but it can also cause damage if it lasts longer than it should. Inflammation that fails to resolve and persists over long periods is known as chronic inflammation. As we get older, such inflammation becomes more common and more harmful, affecting different parts of the body such as the blood vessels, the brain, the joints, and the spine. This can lead to diseases like heart disease, Alzheimer’s disease, arthritis, and chronic pain. At the same time, our immune system, which is responsible for protecting us from germs and cancer cells, becomes weaker and less able to do its job. Scientists are trying to understand how inflammation and immunity change with aging and how to prevent or treat their negative effects.
What is dysbiosis?
The gut microbiome is the collection of bacteria and other microbes that live in our intestines. They help us digest food, fight infections, make vitamins and other substances, and communicate with our brain and other organs. They are important for our health and well-being. Sometimes, the balance of the gut microbiome can be disturbed by factors such as diet, stress, drugs, or diseases – this disturbance is called gut dysbiosis. This can cause problems for our health and increase the risk of obesity, diabetes, inflammation, brain disorders, heart disease, and cancer. Scientists are interested in studying how the gut microbiome changes with aging and how this affects our health.
Can we prevent or cure biological aging in humans?
As of now, there is no scientifically proven method to prevent or reverse biological aging in humans. However, there is also no reason to believe that aging cannot be prevented and/or reversed. The idea of curing aging may sound too good to be true, but many drugs are currently in development that may eventually be used to prevent and/or treat diseases of aging including Alzheimer’s disease (e.g., TauRx) and cancer (e.g., therapeutics based on telomerase inhibitor technology). While medication-based approaches to aging may not be as exciting as alternative (e.g., gene therapy) approaches, these medications are widely believed to be medically practical and may be more likely to give rise to effective interventions for aging in the short term.
Are there any trials underway for treatments to slow or reverse human aging?
Multiple treatments aimed at targets that are thought to underlie the aging process are currently being tested in clinical trials. However, due to the difficulties of measuring aging and lifespan extension in humans, most of these trials focus on the treatment of specific age-related diseases as their primary outcome. A list of ongoing trials arranged according to the mechanism targeted can be found here. A list of ongoing trials and when they are expected to conclude can be found here.
Are there any studies underway that will help us to understand the human aging process better?
Yes. Institutes such as the US National Institute on Aging, the UK National Institute for Health Research, and many more are currently funding research programmes related to the biology of aging. This includes interventions to help prevent/reverse age-related diseases, and improving the detection of early markers of ill health.
What is the difference between chronological age and biological age?
Chronological age is the time that has passed since a person was born. It’s the amount of time they’ve been alive, and it’s the number you’ll find on their birth certificate. Chronological age advances at the same rate for everyone. Biological age, on the other hand, is malleable, shaped by environment and by genetics. The basic idea behind biological age is to approximate what age a person’s body ‘behaves as’ by taking into account how much damage their cells and tissues have accumulated. It is a measure of our overall health and wellness, and is different for each one of us. Your biological age is determined by your body’s ability to function, and includes the state of your organs, circulation, hormones and more.
What is the difference between life expectancy and health expectancy?
Life expectancy is a statistical measure of the average estimated number of years that a member of the human species will live. Health expectancy is a measure of the average estimated number of years that a member of the human species will live in good health, free of chronic disease and disability.
While life expectancy throughout the world has seen a huge rise over the last 100 years, this has been mainly due to a reduction in infant mortality, though medical advances have also played a role. This increase in life expectancy has not been paralleled by an equivalent increase in health expectancy, meaning that a higher proportion of people are now living with chronic diseases of aging that, in the past, would have been fatal to them.
What is the difference between lifespan and healthspan?
Lifespan is the amount of time from birth to death of an individual. Healthspan is the length of time an individual lives in good health. These terms are different from life expectancy and health expectancy, which are statistical measurements of the average expected lifespan and healthspan for a given population.
Human lifespan has greatly increased on average over the last 100 years, but has not been paralleled by an equivalent increase in healthspan, meaning that a higher proportion of people are now living with chronic diseases of aging that, in the past, would have been fatal to them. An effective anti-aging treatment should extend both lifespan and healthspan, thereby increasing the proportion of of one’s life for which one is in good health.
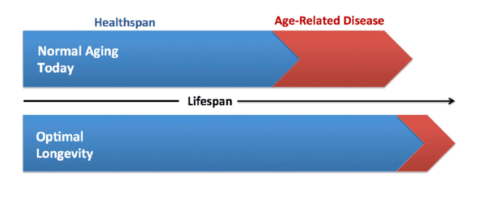
What is the difference between longevity and lifespan?
Many people use the terms “longevity” and “life span” interchangeably. However, when we use these terms in aging research, we usually draw a distinction between the two. Lifespan is simply the amount of time from birth to death. Longevity, on the other hand, is often used as a more comprehensive term referring not only to the ability to achieve an extended lifespan, but to the ability to do so while experiencing limited age-related disease and disability. In other words, the term longevity encompasses not only an extension of lifespan but also the extension of healthspan.
What is the maximum age to which a human can live?
Even if one avoided all fatal diseases, aging would eventually result in the body losing its ability to recover from illness and injury. This means that with current medicine and technology, there is a limit to how long a human can live. Based on mathematical modelling, studies have estimated this age limit at around 140 to 150 years of age.
The oldest human whose age was well documented, Jeanne Calment, lived 122 years and 164 days. As our ability to prevent and treat chronic diseases of ageing improves, we will probably see this age surpassed. However, breakthroughs in our ability to target the fundamental biology of aging may be needed if we are to increase maximum possible human lifespan.
What is the difference between a "genetic mutation" and a "genetic variant"?
A genetic mutation is an alteration in the DNA sequence, which may fundamentally alter or prevent the correct functioning of the protein encoded by that sequence. Mutations can be inherited, and also occur randomly in individual cells throughout the body.
A genetic variant is a polymorphism, which is simply a DNA sequence that appears at the same location within the genome of each individual, but who’s exact sequence varies from person to person. Genetic variants are inherited, and the genetic variants we carry makes each of us genetically unique. Most genetic variants do not increase or decrease an individual’s chances of developing a disease, although some variants have been associated with increased susceptibility to certain diseases of aging like cancer and Alzheimer’s disease.
How do genetic variants contribute to longevity?
Genes are important in regulating several factors that are thought to determine the rate of aging, including nutrient sensing pathways, antioxidant activity, circadian function. Genetic variants can directly contribute to longevity by influencing these processes. Twin studies suggest that together, genetic differences account for roughly 25% of the variance in adult human lifespan.
What is an observational study?
An observational study is a study in which researchers observe data that exists irrespective of the researcher’s intervention. For example, researchers might ask people how much alcohol they drink per week, and then investigate whether there is an association between alcohol consumption and mortality. The researchers are not telling participants of the study how much alcohol to drink – they are simply observing pre-existing drinking patterns.
Observational studies are relatively easy to conduct and recruit for, meaning that it is easier to acquire a large dataset. Observational studies often suffer from selection bias, which is when the selection criteria for the study result in groups that differ from each other in ways besides the variable that the researchers are interested in. For example, people who never drink alcohol are more likely to be former alcoholics, which will make abstinence from alcohol appear to be less healthy than it actually is – these people would need to be excluded from the study. On the other hand, people who drink a lot of alcohol are probably less likely to care about their health, meaning that they are probably less healthy in other ways (less exercise, poor diet and so on). These are confounding factors, and researchers usually attempt to control for them using statistical approaches designed to isolate the effects of the alcohol as much as possible.
It is impossible to control perfectly for every single confounding factor, and so when an observational study shows that a factor (like alcohol) is associated with a certain health outcome, there is always a possibility that the outcome was actually caused by something else (like poor diet). For this reason, observational studies cannot prove causation, only correlation.
What is a randomized, placebo-controlled trial (RCT)?
A randomized trial is a trial in which participants are randomly allocated to one of a number of groups, each of which receives a different intervention or treatment. Researchers then compare the outcomes in the different groups to determine whether the intervention is effective. Randomized trials are often placebo-controlled, meaning that at least one group receives a fake treatment. In a properly placebo-controlled trial, none of the participants are made aware of which treatment they received. Where possible, such trials are also double-blind, meaning that neither the recipient nor the person giving the treatment knows whether the treatment is real or fake.
Because participants are randomly allocated into their respective groups, the composition of the population of each group should be very similar on average – the only significant difference between the groups should be the intervention they are receiving. This eliminates selection bias, which which is when the selection criteria for the study result in groups that differ from each other in ways besides the variable that the researchers are interested in. Randomization means that if researchers observe a difference in outcome between two groups, it is likely to be caused by their intervention and not some other factor. Correlation in a randomized study is therefore more likely to be causal compared with an observational study design.
Randomized, placebo-controlled (and preferably double-blind) trials (RCTs) are the gold standard of study designs, because they are able to establish causation, and are able to rule out the placebo effect as a potential cause of any observed correlation. RCTs are more expensive and harder to recruit for than observational studies, and usually have shorter durations. RCTs are also not always ethical or practical – it’s not ethical to randomize people to smoke cigarettes, for example, and it’s not possible to make a trial of exercise placebo-controlled.
What is Mendelian randomization?
Mendelian randomization is a statistical approach that uses gene variants to help establish whether a given environmental factor causes a particular health outcome, even in a purely observational study.
For example, people who are vitamin D deficient may be more likely to suffer from a bone fracture, but this merely tells us that there is an association between low vitamin D and bone fractures, not that one causes the other. People who get more vitamin D may be more likely to eat healthier foods and do more exercise, for example, and these confounding factors may be responsible for the reduced risk of bone fractures. To establish causation, it would be necessary to perform a randomized trial in which some individuals were selected at random to take more vitamin D, and the effects on bone fractures measured. However, such trials are expensive and not always practical or ethical.
Some people carry gene variants that make them predisposed to have low vitamin D levels, and this is where Mendelian randomization can be useful. Since gene variants are inherited at random from our parents, the distribution of a gene variant within a population can usually be considered to be random (though there are some exceptions to this), and can effectively serve as ‘nature’s randomized trial’. If people with gene variants predisposing them to low vitamin D suffer more fractures, this suggests that low vitamin D does cause fractures, provided we are confident that these gene variants aren’t associated with propensity for exercise, healthy eating, or any other confounding factors.
What is the difference between bias and noise?
Bias is any factor that tends to sway the availability of data, its interpretation, or the data itself in a particular direction, potentially leading us to draw incorrect conclusions. For example, studies with positive findings are more likely to be published, and so there is a publication bias towards studies with positive results. This may lead us to conclude, for example, that an intervention has health benefits, when in reality there is an equal number of studies that show no health benefits.
Noise is the variation within the data that has no meaning or value for the interpretation of said data. For example, suppose you measure your weight once a week. Even if you don’t lose or gain any bodyweight, the result of your weighing will be slightly different each week because you might be more or less hydrated, fed, or wearing heavier/lighter clothes, for example. The direction of this effect is random, and so could either obscure or exaggerate any real change in your weight from week to week – this is noise. If the scales instead gave a reading that always underestimated your weight, they would be biased.
Bias can be mitigated by recognising where it has the potential to occur and either taking precautions to prevent it or accounting for it after the fact. For example, you could use your scales to weigh some objects of known weight. You could then determine by how much the scales underestimated your weight and adjust accordingly. Noise can be mitigated by techniques such as averaging. For example, rather than weighing yourself once, you could weigh yourself multiple times throughout the day and calculate the average. Some statistical methods may also be able to reduce noise from a set of data.
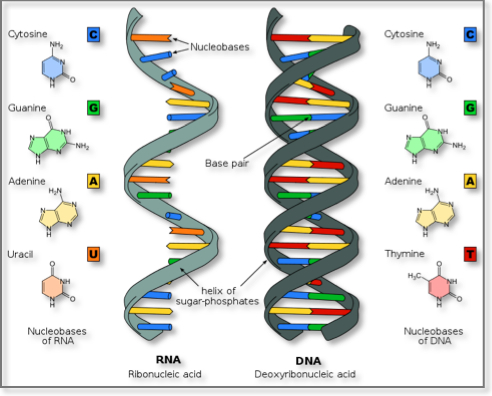
What is the difference between DNA and RNA?
DNA and RNA are both molecules that contain genetic information. The chemical structure of RNA is similar to that of DNA: both are made up of nucleotides, each consisting of a phosphate group, a ribose sugar and a nucleobase, the latter being the variable component of which the sequence forms the genetic code. In DNA, the sugar molecule is deoxyribose while RNA instead has ribose. Additionally, the nucleobase thymine (T) in DNA is replaced with uracil (U) in RNA. Unlike DNA, which is usually found in its double-stranded helix structure, RNA is usually found as single unpaired strands, though exceptions exist for both cases.
In humans, DNA serves the role of a permanent, mostly unchanging copy of the individual’s genome that is maintained within the nucleus of each cell. RNA fulfils many roles within human cells and has many subtypes. The most important functions of the RNA are to serve as a temporary copies of genetic code found within the DNA, and to orchestrate the assembly proteins using these copies. In this way, genetic information can be transmitted from within the nucleus to the sites of protein assembly without the DNA ever needing to leave the safety of the nucleus. There are however viruses that use RNA to store their genetic information. The common cold, influenza, COVID-19 and measles are examples of RNA viruses.
What are the main subtypes of RNA?
Messenger RNA (mRNA) serves as the messenger between the DNA and the ribosomes – the cell’s protein factories. Within the nucleus, a section of DNA coding for a protein is used as a template to create a strand of mRNA in a process called transcription. This mRNA then exits the nucleus and travels to the ribosome, where it is used to build the protein encoded by the original DNA sequence.

DNA is transcribed into mRNA, which is then translated into proteins at the ribosome.
Source
Ribosomal RNA (rRNA) is the RNA that forms an essential component of the ribosomes – the cell’s protein factories. rRNA makes up about 80% of all cellular RNA. Unlike mRNA, rRNA is non-coding RNA, meaning that it is never translated into a protein. Rather, it plays a structural role within the ribosome, and serves to bring the messenger RNA and the transfer RNA together so that proteins can be built.
Transfer RNA (tRNA) is responsible for bringing the amino acids – the building blocks of proteins – to the ribosome to be joined together in a process called translation. Every three letters (or codon) of messenger RNA sequence encodes a specific amino acid. Each tRNA molecule comes attached to an amino acid at one end. At the other end, there is a three letter sequence that is complementary to the triplet which encodes that amino acid. rRNAs line up with the triplets of the mRNA, bringing their amino acids together to be joined into a protein by the ribosome.
Translation of mRNA into a protein at the ribosome.
An Introduction to Ribosomes: Nature’s busiest molecular machines
Viral RNA is found within RNA viruses. Some viruses (such as those that cause influenza, measles and COVID-19) encode their genetic information within RNA rather than DNA. Most viral RNA is single-stranded RNA, though this is not always the case.
Micro RNA (miRNA) and Small Interfering RNA (siRNA) are small RNAs which don’t code for proteins, but instead have the ability to neutralise complementary mRNA molecules by binding to them in a process called silencing, making them a form of post-transcriptional regulation of gene expression.
What is ‘inflammaging’?
Inflammaging is a term used to describe the age-related overactivation of the innate immune system, which is responsible for the inflammatory response. Inflammation is a vital process for the immune system to function as a defence against pathogens, but is also harmful to the body’s own tissues. Inflammation is an important factor in driving most age-related diseases. This article provides a more detailed explanation of inflammation and it’s relationship with the aging process.
What is the relationship between mitochondria and the aging process?
The mitochondria, which are the power stations of the cell, produce an energy-storing molecule known as ATP. This molecule is used to power chemical reactions and processes that are essential for life. As cells age, their mitochondria become less efficient at producing ATP, which may play into some age related diseases. Mitochondria are also important in other ways: they regulate various processes in the cell through molecular signals they release, play a key role in apoptosis (cell suicide), and are also a major source of reactive oxygen species. This article explores mitochondria and their relationship with aging in greater detail.
What are free radicals, reactive oxygen species and oxidative stress?
Free radicals are highly reactive molecules that can damage other molecules by ‘stealing’ electrons from their atoms. Reactive oxygen species or ROS are free radicals that are derived from oxygen, while oxidative stress is the term used to describe the damage sustained by cells due to the production of ROS. ROS are a natural byproduct of normal cellular function. Inflammation also generates ROS. Many environmental factors such as cigarette smoke, radiation, and high fat, high sugar diets can lead to increased oxidative stress.
What are antioxidants?
Antioxidants are molecules that neutralise reactive oxygen species by ‘donating’ one of their electrons. Antioxidants are found in abundance in many plants-based whole foods. Many studies report an association between antioxidant-rich diets and longevity, but whether antioxidants actually slow aging in humans is debated.
What is the relationship between diet and longevity?
In addition to the effects of unhealthy diets and obesity on the risk of developing diseases of aging, many studies suggest that limiting calorie intake and/or reducing the levels of glucose, insulin, or fatty acids in the blood can extend maximum life span. Although it is not yet clear whether and by how much dietary restriction alone can increase life span in humans, several groups have demonstrated that dietary modifications can increase both health span and life span in laboratory models.
It is estimated that 25% of the variation in adult human lifespan is the result of genetic factors. It is clear that environmental factors play an important role, but it is difficult to separate the effects of diet specifically from those of other factors, particularly socio-economic status. We can say that there is an association between low-calorie, antioxidant-rich diets and longevity. Extreme examples of this include the Okinawan diet, which is vegetable and fruit heavy, but low in meat, refined grains, saturated fat, sugar, salt, and full-fat dairy products.
Can injecting blood from a young person make you younger?
Joining the circulatory systems of an old mouse and a young mouse (a process called parabiosis) can rejuvenate the tissues of the older mouse. However, it is not certain whether this is the result of rejuvenating factors in young blood, or whether it is caused by dilution and removal of harmful waste products by the younger animal’s organs. Repeated infusions of young blood can rejuvenate mice, but so can diluting the blood with a solution of saline and albumin (the main protein component of blood plasma). The effects of such treatments in humans are not yet well established, but there are some early indications that they may be effective for treating some age-related diseases. Clinical trials are ongoing. For a more detailed explanation and list of clinical trials, see this article.
What are metabolism and metabolic rate?
Metabolism refers to the complex series of biochemical reactions that convert the calories you consume into usable energy. Metabolic rate is the rate at which this energy is consumed by the body. Metabolic rate is determined by a number of factors including gender, age, muscle mass and physical activity. This article provides a more detailed explanation of metabolic rate and what constitutes a desirable metabolic rate.
What is amyloid?
Amyloid is formed from misfolded proteins that aggregate together to form fibrous deposits called amyloid plaques. Prominant examples include amyloid beta in Alzheimer’s disease and alpha synuclein in Parkinson’s disease. Amyloid plaques can form in the brain and are linked to neurodegenerative diseases, but can also form in organs throughout the body and contribute to other age related diseases, such as diabetes and heart disease. This article provides a more detailed explanation of amyloid, where it comes from, and what you can do about it.
What is insulin?
Insulin is a hormone released by beta cells in the pancreas in response to blood sugar (glucose) levels. Insulin has multiple effects relating to glucose and acts to lower blood sugar.
- Insulin increases the movement of glucose from the blood and into cells throughout the body.
- Insulin promotes the conversion of glucose into glycogen stores (glycogenesis), mainly in the muscle and the liver.
- Insulin promotes the conversion of glucose into fat in the liver (lipogenesis).
- Insulin inhibits the production of new glucose by the liver (gluconeogenesis).
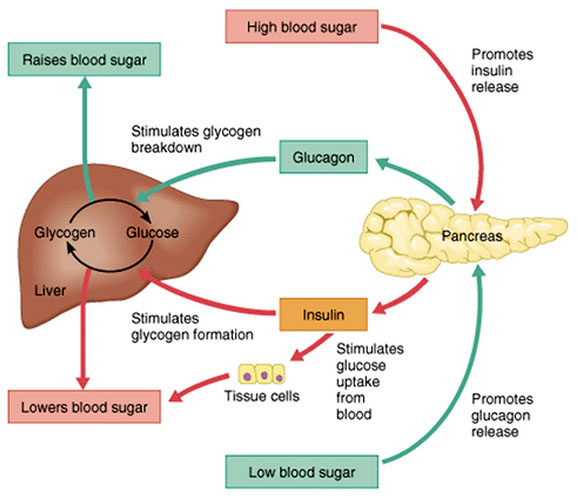 Diagram showing some of the effects of insulin (and its antagonistic hormone, glucagon)
Diagram showing some of the effects of insulin (and its antagonistic hormone, glucagon)
Source
What is insulin resistance?
Insulin resistance is a metabolic disorder in which cells do not respond as strongly to the blood sugar-lowering hormone insulin, meaning that more insulin must be released in order to produce a given reduction in blood sugar. Following the consumption of carbohydrates, the cells of a person with insulin resistance do not absorb as much glucose from the blood. At the same time, insulin fails to block the production of new glucose in the liver. Consequently, the blood sugar of an insulin resistant person may remain higher for an extended period of time compared with a healthy person. More severe insulin resistance can result in fasting hyperglycaemia, in which blood sugar remains elevated after 8 hours without food or drink.
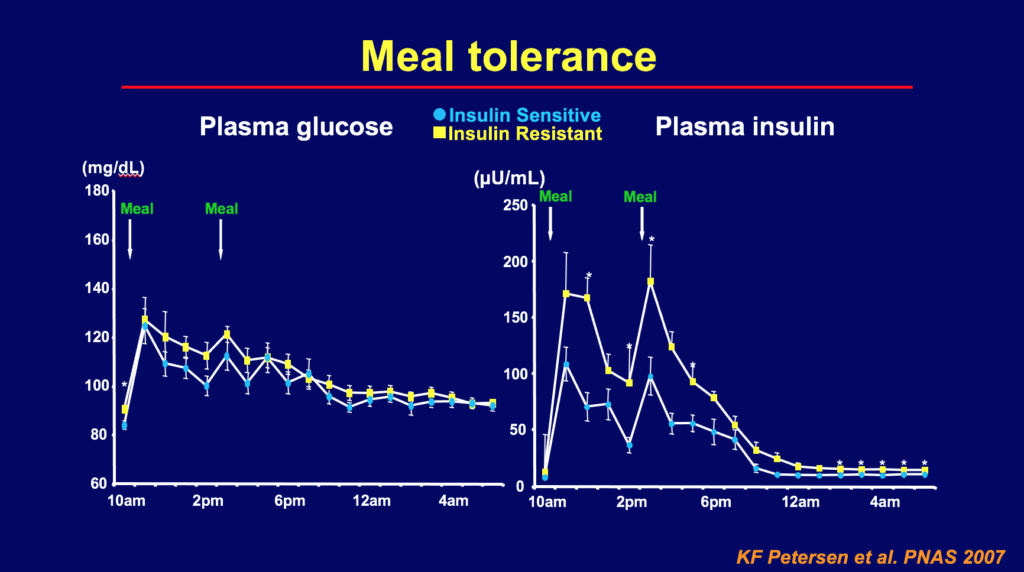
Glucose and insulin levels following consumption of a high-carbohydrate milkshake. Image credit: Gerald Shulman
Source
While most commonly associated with type II diabetes mellitus, insulin resistance contributes to a wide range of chronic diseases including heart disease, neurodegenerative diseases, fatty liver disease and cancer. It is possible to be insulin resistant without suffering any negative health consequences (at least initially). Many people are probably not aware that they are insulin resistant. For a much more detailed guide to insulin resistance and how it happens, take a look at this article.

Insulin resistance drives or is implicated in a diverse range of diseases.
Source
How is aging related to Alzheimer's disease?
Alzheimer’s disease is one of a handful of diseases that generally affect only older individuals and account for a disproportionate share of deaths in the elderly. Alzheimer’s disease is linked to the accumulation of misfolded proteins to form amyloid plaques in the brain. It is hypothesised that these plaques lead to a loss of neural function, though the truth may be more complex. Amyloid accumulates in old age because cells become more prone to producing misfolded proteins, and because inflammation promotes the misfolding of correctly folded proteins outside of the cell. However, research suggests that Alzheimer’s disease is not an inevitable consequence of old age.
Are all people equally likely to get Alzheimer's disease?
No. Genetic factors play a large role in the risk for Alzheimer’s disease, but there are also a number of lifestyle factors (e.g., diet, physical activity) that may influence the risk for developing Alzheimer’s disease later in life.
The gene with the strongest impact on Alzheimer’s risk is the APOE gene. Of the three most common APOE variants, APOE-e4 is most strongly associated with the disease. Around 40-65% of Alzheimer’s sufferers have one or two copies of the APOE-e4 gene. Inheriting two copies, one from each parent, increases the risk of developing Alzheimer’s.
Other factors that increase the risk of developing Alzheimer’s disease include smoking, high blood pressure, high cholesterol, diabetes and cardiovascular disease.
What is the difference between dementia and Alzheimer's Disease?
Dementia is an umbrella term for a variety of conditions that result in impaired mental function. Alzheimer’s disease is the most common type of dementia. Other forms of dementia include Lewy Body dementia (associated with deposits in the brain called Lewy bodies), Parkinson’s disease, vascular dementia (associated with small vessel strokes), and frontotemporal dementia (associated with a degeneration of neural structures in the frontal and temporal lobes).
How is aging related to Parkinson's disease?
Parkinson’s disease is a degenerative disorder of the nervous system caused by the death of dopamine-secreting neurons in the substantia nigra region of the brain. Parkinson’s disease is most common in older individuals. Like Alzheimer’s disease, it is linked to the formation of amyloid plaques, in this case composed of a misfolded protein called alpha-synuclein.
Does aging cause cognitive decline?
Yes. Cognitive decline with aging is a normal part of the aging process. As people age, they tend to have more difficulty learning new information, concentrating for long periods of time, and suffer a decline in memory function. Decline in cognitive function is one of the most important contributors to loss of independence and quality of life among the elderly.
How is aging related to Cancer?
Cancer is the result of abnormal and malignant cell growth, which is caused by the accumulation of various genetic mutations. Although cancers can occur in the young, the rate at which they are diagnosed increases exponentially with age. This is because the DNA in older individuals is exposed to more sources of damage, while DNA repair mechanisms become less effective.
How does aging affect the muscular system?
During aging, the muscle stem cells responsible for regenerating muscle tissue become less able to multiply. Combined with changes in growth signals, this leads to a decline in skeletal muscle mass and strength. The most extreme form of this is sarcopenia, a syndrome characterized by progressive loss of skeletal muscle leading to physical disability, poor quality of life, and increased mortality. Age related muscle loss is worsened by a sedentary lifestyle, while exercise can reverse it to some extent.
 .
.
How does aging affect the skeleton?
During aging, cells that build new bone tissue become less active, while the activity of cells that break it down increases. This leads to a loss of bone mass beginning around middle age, making bones more fragile. This can eventually lead to osteoporosis, a severe weakening of the bones that is associated with more frequent fractures and therefore potentially chronic pain.
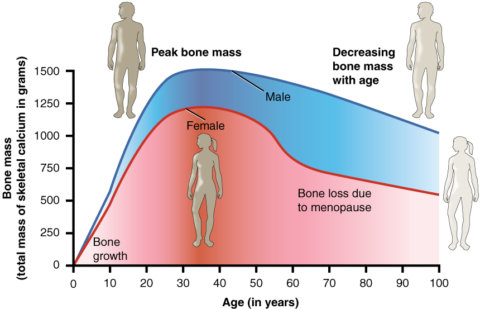
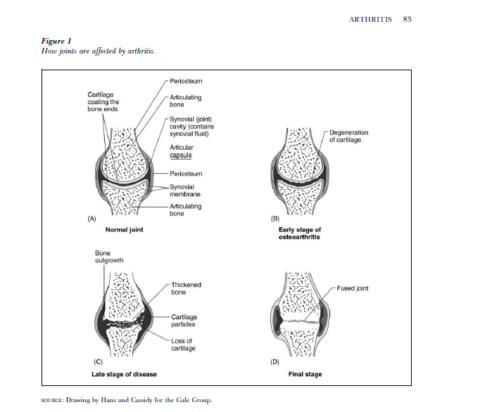
Within the joints, cartilage that protects the ends of the two bones can degenerate with age. The loss of this tissue exposes the bone beneath, which begins to be eroded as well, leading to osteoarthritis.
Bone aging is worsened by lack of exercise (exercise stimulates the cells that build bone tissue), and by lack of certain nutrients, especially calcium and vitamin D.
How does aging affect the circulatory system?
With age, the walls of blood vessels become thicker and stiffer, resulting in an increase in blood pressure. This makes the blood vessels more vulnerable to damage, and puts more strain on the heart. The heart may grow larger, either because the walls are thickened (meaning the ventricles hold less blood) or because they are stretched (making contraction weaker).
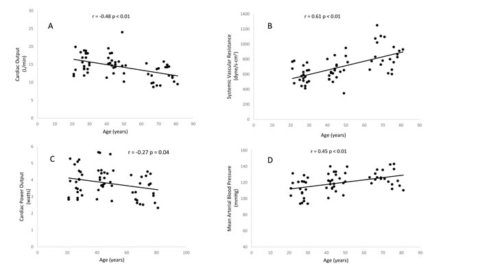
Image source: http://dx.doi.org/10.18632/oncotarget.12403
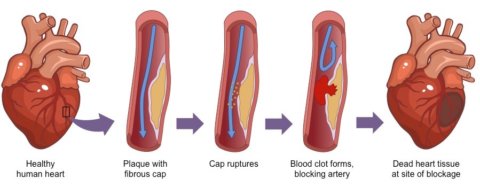
Arteries may develop atherosclerotic plaques, in which fatty material builds up in the artery wall. This narrows the artery, further increasing blood pressure. This plaque may cause the formation of a blood clot that blocks the artery, which may lead to a heart attack if it occurs within the heart. The plaque may also break away and become lodged elsewhere, resulting in an embolism.
Risk of heart disease is heavily influenced by physical activity, diet, and smoking.
How does aging affect the endocrine (hormonal) system?
Production of most hormones declines with age, and even for those that don’t, hormone receptors usually become less sensitive. Notable hormones that decline with age include oestrogen and testosterone (related to a decrease in reproductive health), growth hormone (related to decreased muscle mass and regenerative capacity), and melatonin (related to sleep loss).
The thyroid gland produces less of the thyroid hormones with age, which leads to a gradual decrease in metabolic rate. This leads to less heat production and increased body fat.
Insulin sensitivity declines with age, and insulin levels may decrease, contributing to an increased risk of diabetes.
How does aging affect the skin and hair?
With age, the skin becomes thinner and less elastic due to a decline in connective tissue components collagen and elastin, and due to cross-linking of these proteins by molecules called advanced glycation end products. This makes the skin more fragile and results in the formation of wrinkles. Aged skin also takes longer to heal.
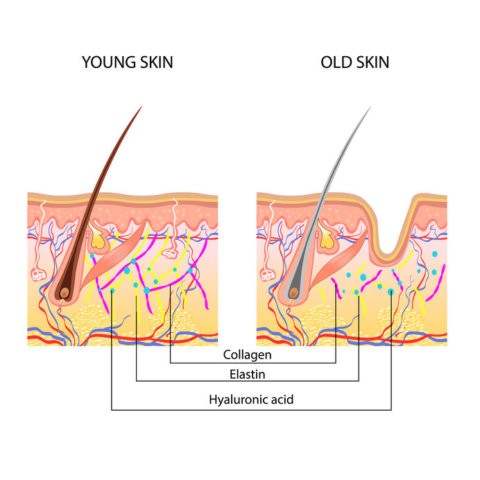
Anatomical Difference Between Young And Old Skin
Aging can result in hair loss, though this is far more significant in males, and can happen for different reasons. With age, individual hairs spend less time in their growth phase (see here for an explanation of how hair grows), and hair follicles become smaller and eventually die.
Hair also becomes grey with age. This is caused by the death of follicular melanocytes, the cells that produce the pigment responsible for hair colour.
How does aging affect the digestive system?
With age, the stomach’s lining becomes less resistant to damage, which may increase the risk of peptic ulcer disease. With age, the stomach becomes less elastic and cannot accommodate as much food.
Due to changes to the structure, enzyme secretion, and bacterial populations in the small and large intestines, nutrient absorption may decrease. Levels of the enzyme lactase decrease, which may lead to intolerance of dairy products (lactose intolerance).
Some structural changes occur in other components of the digestive system, but these do not significantly impact the digestive process.
How does aging affect the immune system?
With age, the immune system becomes less effective at protecting the body against pathogens. The adaptive immune system (the part of the immune system that recognises specific pathogens) declines in favour of the innate immune system (which is more involved in inflammation). This results in increased susceptibility to chronic inflammatory diseases. These changes happen for a variety of reasons, including:
- Hematopoietic stem cells in the bone marrow decline in number and become less likely to develop into lymphocytes (such as T cells, B cells) and more likely to produce cells of the myeloid lineage (like macrophages and neutrophils).
- The thymus, the organ in which T cells mature, shrinks with age. This contributes to a decline in the number of naive T cells (T cells that have not yet encountered foreign antigens and are available to respond to previously unseen pathogens).
- Naïve T cells are further depleted by repeated viral infections.
- Senescent cells throughout the body release inflammatory molecules that contribute to the overactivity of the innate immune system.
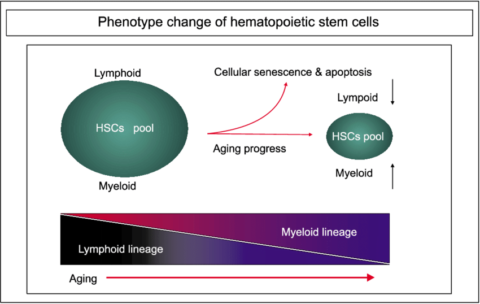
The overall result is that the aging immune system takes longer to respond to pathogens, is less effective at removing them, and is more likely to produce excessive and harmful inflammatory responses.
How does aging affect the brain and the rest of the nervous system?
With age, the brain shrinks in volume and becomes more susceptible to strokes due to blood vessel aging and increased blood pressure. Some neurons may die, but it is not known to what extent this matters in the aging brain. Certain neurotransmitters, such as dopamine and serotonin, decline. The formation of amyloid plaques in the brain may kill cells, cause inflammation, and result in neurodegenerative diseases such as Alzheimer’s disease.
Normal brain aging is associated with a decline in cognition, particularly a loss of memory function.
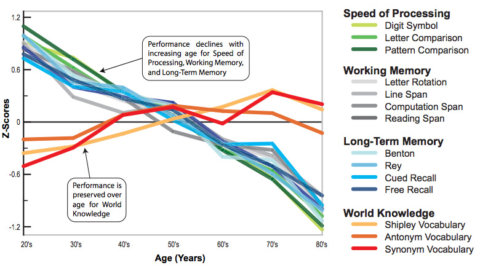
Nerves may conduct signals more slowly due the deterioration of the myelin sheath, the protective covering that surrounds some nerve fibres. In the spine, disks between the vertebrae become harder, and parts of the vertebrae may overgrow. This puts more pressure on the nerves that exit the spinal cord, which can sometimes result in reduced sensation and impaired balance.
How does aging affect the kidneys?
Nephrons (the structures in the kidney that filter the blood and reabsorb/secrete molecules as necessary) atrophy and decline in number. This is thought to be partly caused by an impared oxygen supply due to the stiffening of arteries.
Glomerular filtration rate (the rate at which blood is filtered by the kidneys) declines by roughly 50% between the ages of 30 and 90 in healthy individuals. However, this is thought to have relatively little effect on life expectancy.
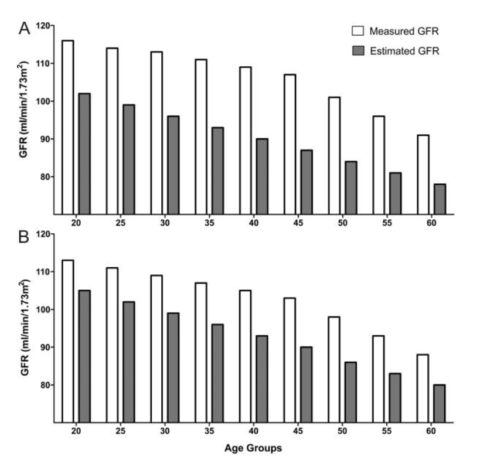
Glomerular filtration rate in (A) females and (B) males (source).
Age-associated increases in blood pressure, cholesterol, and inflammation increase the risk of chronic kidney disease (in which the kidneys do not filter the blood quickly enough and dialysis/kidney transplant may eventually be needed).
How does aging affect the liver?
With age, the liver decreases in overall size and blood flow is reduced. The liver becomes less efficient at metabolising many substances, meaning that drugs may not be inactivated as quickly in older people. Metabolism of LDL cholesterol decreases, leading to an increase of cholesterol in the blood. The aging liver also becomes less resistant to damage from toxins, and this damage takes longer to repair than in younger people.
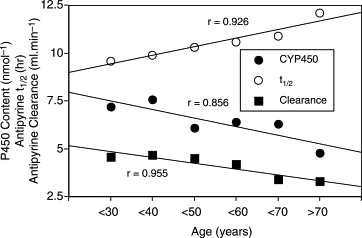
Changes in antipyrine clearance (a measure of liver function) and CYPs (liver enzymes) with age (source).
Production of bile decreases with age, increasing the risk of gallstone formation.
Age-related changes in cholesterol, insulin secretion, fat distribution, and inflammation may increase the risk of non-alcoholic fatty liver disease and liver cirrhosis.
How does aging affect the lungs?
The lungs lose some of their elasticity with age, and the thoracic cage undergoes some structural changes. This means that the respiratory muscles must generate more pressure in order to produce a given change in lung volume (this is called reduced compliance).
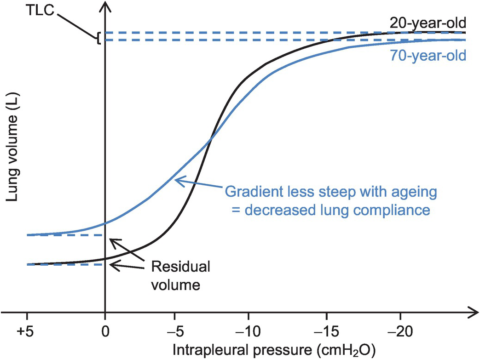
Lung compliance in a 20 year-old compared with a 70 year-old (source).
Calcification of the cartilage, osteoporosis, and weakening of the respiratory muscles reduce the ribcage’s ability to expand and contract.
The presence of antioxidants within alveoli (where gases are exchanged with the blood) decreases, while persistent inflammation within the lung increases. This can injure the alveoli, causing gas exchange to become less efficient. The result of these changes is a reduction in overall lung function and exercise capacity. Older lungs have a lower maximum rate of oxygen exchange and cannot expel air as quickly.
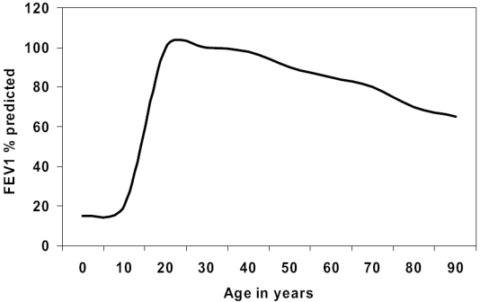
Age related change in forced expiratory volume in 1 second (the maximum volume of air that can be expelled from the lungs in one second) (source)
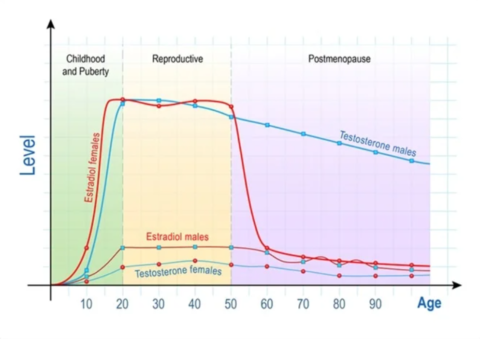
How does aging affect reproductive health?
Both males and females experience a decline in the production of sex hormones into old age. The majority of individuals report a decline in sex drive with age, though it is not clear to what extent this is due to hormonal factors vs psychological factors.
In females, the number of follicles (the small sacs within the ovaries that release eggs) declines with age. This decline increases sharply following the menopause, when ovulation and the menstrual cycle cease.
Although ovulation stops, the rest of the reproductive system remains functional enough that postmenopausal women can still become pregnant via donor eggs and give birth.
The function of the placenta and the muscles of the uterus and cervix decline with age, contributing to the risk of complications during pregnancy. Other age-related changes such as increased blood pressure also contribute.
In males, the rate of production, motility and viability of sperm decreases with age, and this appears to be associated with difficulties conceiving and potentially lower live birth rates in the case of IVF.
With age, the rate of DNA mutations within sperm cells increases, which raises the risk of genetic disorders in the offspring.
Hormonal changes during aging commonly result in benign prostatic hyperplasia (enlargement), which may lead to difficulties urinating.
How is aging related to cardiovascular disease?
Cardiovascular disease refers to a group of chronic diseases that affect the heart and blood vessels. Risk factors for cardiovascular disease include high blood pressure, high cholesterol, obesity, tobacco use, and physical inactivity. Most cardiovascular disease is ultimately driven by dysfunction of the endothelium (the inner lining of the blood vessels) and by chronic inflammation, both of which increase in old age.
How does aging affect fertility?
As humans age, they are less fertile. Women experience a decline in fertility starting at around age 30. Men experience a decline in fertility starting at around age 40. As people age, they become increasingly at risk of developing infertility.
What is the link between sleep and the aging process?
Advanced age is associated with a decline in sleep duration and sleep quality. We are not certain to what extent aging causes sleep loss or to what extent sleep loss drives the aging process: both statements may be true to some extent.
Poor sleep quality appears to accelerate ageing at the cellular level: sleep loss is associated with shorter telomeres and possible increased cellular senescence, both of which are key mechanisms underlying the ageing process. Reduced sleep quality has been linked to increased risk of developing chronic diseases such as Alzheimer’s disease. Some evidence suggests sleep may play a role in clearing the brain of toxic proteins that drive neurodegenerative diseases.

(image source).
What is geriatric medicine?
Geriatric medicine is the branch of medicine that focuses on the medical problems of older individuals. Geriatricians have had additional training in the diagnosis and treatment of diseases common in older patients. Most commonly, geriatrics refers to an specialty of care for the elderly, although geriatricians can be any physician who chooses to specialise in treating aging-related diseases.
What is gerontology?
Gerontology is the scientific study of aging processes.
Can you predict how long someone will live?
It is not possible to predict exactly how long an individual will live with 100% certainty. Globally, the average life expectancy for a member of the human species born in 2020 is estimated at 70 years for males and 75 years for females). However, many individuals live well past 100 with a delayed onset or reduced severity of age-related conditions. It is not clear exactly what environmental or genetic factors are important when it comes to achieving exceptional longevity.
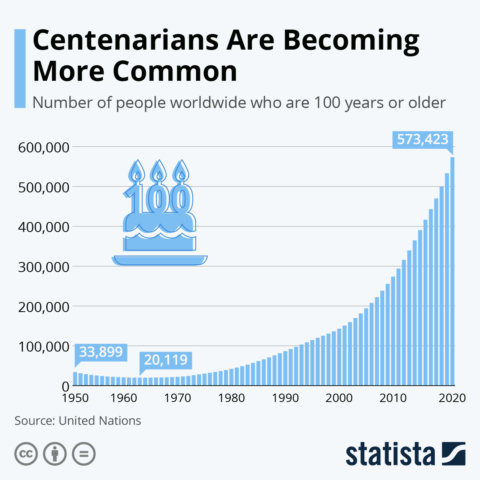
What are centenarians?
A centenarian is an individual who lives to reach the age of 100. There are over 500,000 centenarians worldwide, and this number is increasing. The country with the highest number of centenarians per person is Japan, where approximately 6 in every 10000 people are 100+ years old.
What are the major causes of death?
The world’s two leading causes of death are heart disease, which killed 17.8 million people in 2017, and cancers, which killed 9.6 million that year. The third biggest killers were respiratory diseases, killing 3.9 million. However, all of the above are actually groups of diseases. If we consider specific diseases, then ischemic heart disease is the biggest killer globally, followed by stroke and chronic obstructive pulmonary disease.
What are the major causes of disability requirements for long-term care?
Cancer and heart disease contribute to the majority of disability among older Americans. Both of these conditions progress slowly and can be managed with medications and lifestyle changes. Cancer is often accompanied by depression, anxiety, and other mood disorders that can have a substantial negative impact on quality of life. Heart disease can cause difficulty walking or climbing stairs, as well as shortness of breath.
Are there supplements for improving your longevity?
There are many supplements that may be beneficial to health and that act through mechanisms thought to be related to aging, such as by reducing oxidative stress. However, there is no evidence that any of these supplements can extend human lifespan. Any suggestion that supplements can effect the progression of aging is largely speculative and based on short term studies measuring biomarkers of aging. Some common supplements include vitamins, minerals and antioxidants. Supplements may have the most positive health impact when they address existing insufficiencies, which may become increasingly common with age as the gut becomes less effective at absorbing nutrients.
Can drinking more coffee increase longevity?
Possibly. Meta-analyses suggest that coffee consumption was associated with a reduction in all-cause mortality of up to 17% in men and 37% in women. Consuming larger quantities of coffee was associated with greater reductions in mortality. Studies have suggested that coffee consumption may reduce the risk of specific diseases of aging such as heart disease and cancer, but not all research is in agreement and some data is likely to be biased.
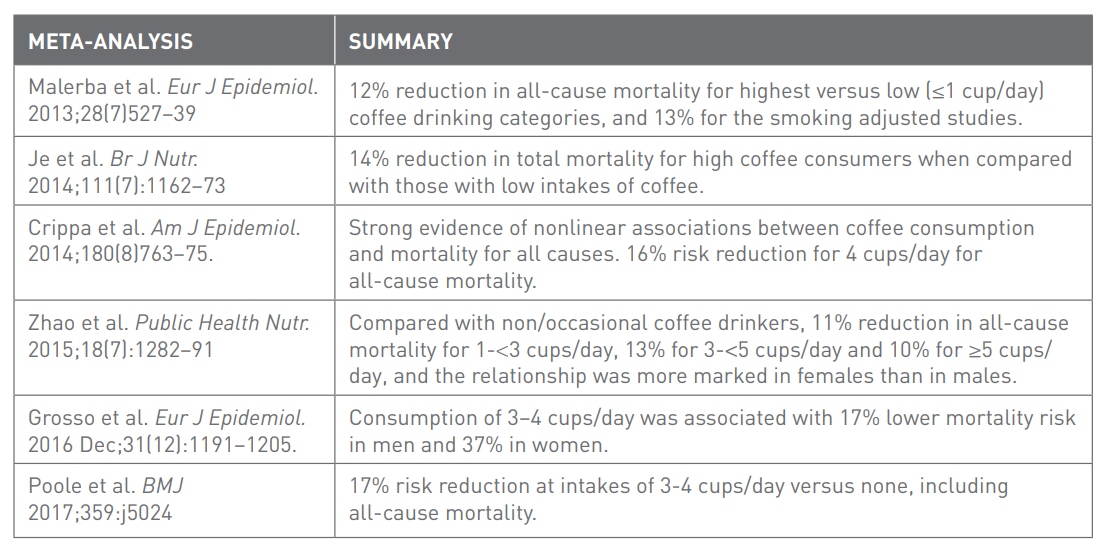
A summary of the findings from 6 meta-analyses of the effects of coffee consumption on mortality (Source)
While there is good evidence that caffeine can improve life expectancy, caffeine alone is unlikely to fully explain the effects of coffee. It is thought that antioxidant and anti-inflammatory compounds within coffee (such as polyphenols) are likely to play a significant role.
Can green tea or its extract increase longevity?
Possibly. A study of 100 000 Chinese adults found that those who drank green tea 3 times a week or more lived on average one year longer at age 50.
An analysis of 13 studies found a significant reduction in both blood pressure and cholesterol with consumption of green tea or its extract. Other meta analyses suggest that green tea consumption reduces the risk of breast cancer and prostate cancer. Green tea consumption is also associated with reduced risk of neurodegenerative disease, reduced risk of respiratory infection, and improved weight loss.
While there is good evidence that caffeine can improve life expectancy, caffeine alone is unlikely to fully explain the effects of green tea. It is thought that antioxidant and anti-inflammatory compounds within green tea (such as catechins) are likely to play a significant role.
Can black tea increase longevity?
Possibly not. A study of 100 000 Chinese adults did not find a significant relationship between black tea consumption and reduced mortality or reduced risk of heart disease.
Black tea contains fewer antioxidants when compared with green tea, which may explain why it does not appear to share green tea’s association with increased longevity. It is also possible that black tea is more habitually consumed alongside other dietary components with negative health consequences, which may counter the beneficial effects of black tea.
Can ginger supplements slow aging?
According to this review of the evidence, ginger may be effective in slowing the progression of certain age-related diseases in both animals and humans. Here is a summary of their conclusions:
- In animals, ginger appears to slow the progression of ageing in several organs, including the heart, the lungs and the brain.
- Studies support the idea that ginger supplementation can improve cognitive function in both animals and elderly humans.
- Ginger appears to protect animals against Parkinson’s disease and Alzheimer’s disease, but whether this holds true in humans is unclear.
- Ginger supplementation appears to be beneficial for patients with type 2 diabetes (by improving the response to sugar), rheumatoid arthritis and osteoarthritis (by reducing inflammation and relieving pain).
- In animal models, ginger can protect against heart disease by lowering blood pressure, ‘bad’ cholesterol (LDL) and inflammation, and results in a reduction in the size of fatty deposits in the arteries. There is evidence that at least one of these effects (namely blood pressure reduction) extends to humans as well.
Can vitamin B3 (niacin) slow aging?
It is theorised that vitamin B3 might be able to slow aging by increasing levels of a molecule called nicotinamide adenine dinucleotide (NAD). NAD levels decline with age, and preliminary evidence suggests that restoring NAD may reverse some aspects of ageing.
Studies suggest that vitamin B3 can normalise blood lipid levels in people with high LDL cholesterol or low HDL cholesterol. However, a meta analysis of 35 760 subjects found no beneficial effect for vitamin B3 in the treatment of cardiovascular disease.
Some studies report that application of vitamin B3 can improve the appearance of aging skin.
Can berberine supplementation protect against diseases of aging?
Berberine is extracted from several different plants, and has been used in traditional Chinese medicine. It may protect against a range of chronic disease of aging, primarily by protecting against elevated blood sugar levels and insulin resistance, which is a major driver of many chronic diseases including diabetes, heart disease, cancer and fatty liver disease. In type II diabetics, berberine is one of the few supplements found to have similar effectiveness when compared to pharmaceutical drugs such as metformin, though the effects of berberine are still less well supported by research than most pharmaceuticals.
This article gives a more detailed overview of berberine and the evidence supporting its effects on blood sugar. Berberine may also aid weight loss, slow tumour growth, and protect against certain pathogens.
Berberine has a high potential to interact with medication, causing severe reactions in some cases.

Can licorice extract protect against diseases of aging?
Research suggests that daily consumption of licorice extract for 6 months is associated with reduced oxidative stress and a 20% reduction in the oxidation of low density lipoprotein, which is a key driver of atherosclerosis. In line with this, consuming 0.2 g/day of licorice extract for 12 months appears to slow the progression of atherosclerosis in those with elevated cholesterol.
Can magnesium supplements protect against diseases of aging?
A meta-analysis of 22 trials involving 1173 participants concluded that magnesium supplementation achieved a small but clinically significant reduction in blood pressure, and may therefore protect against heart disease. This reduction was most significant in those who were magnesium deficient or had elevated blood pressure. There is also evidence from meta-analyses that magnesium supplementation can improve insulin sensitivity and reduce blood sugar in those with or at risk of diabetes, though the blood sugar reduction is not always reliable.
Some studies suggest that magnesium supplementation may improve sleep quality in people who sleep poorly. Improved sleep quality is associated with reduced risk of developing neurodegenerative diseases such as Alzheimer’s.
Can you increase your lifespan by eating a healthy diet?
Yes. A diet that is rich in raw fruits, vegetables, whole grains, and fibre is associated with a decreased risk for developing a number of chronic diseases of aging such as heart disease, diabetes, and cancer. However, it is difficult to gauge how much of an impact diet has on longevity in humans, due to the difficulty of separating the effects of diet from those of other factors like socioeconomic status.
Can you increase your lifespan by exercising?
Yes. Even a small amount of moderate intensity exercise helps to prevent the development of chronic diseases of aging such as heart disease. Although confounding factors (like diet, education and location) are challenging to control for completely, research suggests that leisure-time activity equivalent to up to 75 minutes/week of brisk walking was associated with 1.8 years of life gained compared with no leisure time activity. 450+ minutes/week was associated with 4.5 years of life gained. Unfortunately, age-related changes tend to make exercise more difficult.
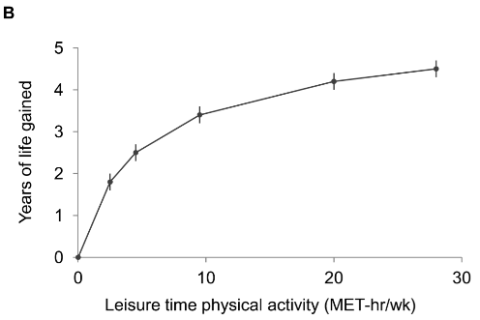
Can 'detox' treatments increase your lifespan?
Detox methods don’t help the body remove toxins, though some of them might increase your lifespan in other ways. The liver and kidneys are perfectly capable of removing toxic substances from the body without need of assistance. The only exceptions to this are when these organs are damaged, when a substancial quantity of toxins are ingested (such as exessive alcohol consumption), or in the case of certain rarely encountered chemical that take a long time for the body to clear (such as heavy metals and some organic polluants). However, there is no evidcence that detox methods help improve the body’s ability to clear these toxins. Some detox practices (such as fasting and exercise) can nevertheless improve your health longevity. This article explains in more detail what constitutes a toxin and how the body removes them.
What are telomeres?
Telomeres are the “caps” on our chromosomes that protect chromosome ends from damage. Each time a cell divides, telomeres shorten. When telomere length gets too short, it signals the cell to stop dividing and go into senescence (senescent cells cannot divide and accumulate with age). Quantifying telomere length is an objective way to quantify biological aging – individuals with longer telomeres tend to be biologically younger than others of the same age.
Read all our articles on telomeres and its impact on health and longevity.
What is telomere length?
Telomeres are DNA-protein complexes at the end of chromosomes that protect chromosomes from damage by binding to proteins called shelterin. Telomeres are shortened each time a cell divides. As the telomere repair system becomes less efficient and synthesis of new telomeric DNA decreases, cells enter a state of replicative senescence (telomeres become too short to protect chromosomes during cell division). Replicative senescence in human skin fibroblasts has been shown to correlate with age; average telomere length decreases by approximately 30 base pairs per year.
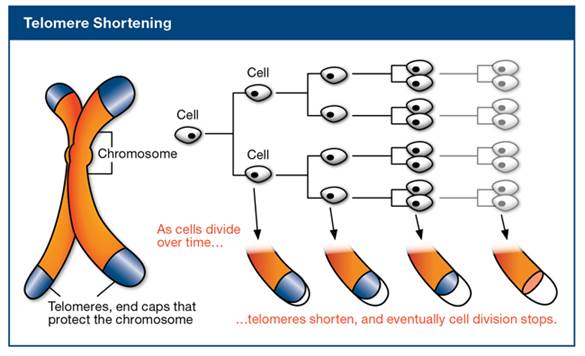
As the telomere repair system becomes less efficient and synthesis of new telomeric DNA decreases, cells enter a state of replicative senescence (telomeres become too short to protect chromosomes during cell division). Replicative senescence in human skin fibroblasts has been shown to correlate with age; average telomere length decreases by approximately 30 base pairs per year. When telomere length is too short, the cell becomes “senescent” and stops dividing. Senescent cells are associated with age-related diseases, including heart disease, cancer, arthritis, and cognitive decline.
Is there a way to extend telomere length?
Extending telomeres would require changing the rate at which they shorten during cell division or activating mechanisms that repair and elongate damaged telomeres. Telomeres are shortened by DNA damage, so one possible hypothesis is that preventing DNA damage may extend telomere length. In fact, many laboratory studies have shown that under certain circumstances, dietary supplements (e.g., resveratrol) or specific types of nutrients (e.g., vitamin D) can help prevent DNA damage and may therefore be able to extend telomere length. However, it has been difficult to confirm these results in humans. There’s also a lot more to aging than telomere length, and it’s not clear by how much extending telomere length would increase human longevity.
What is sarcopenia?
Sarcopenia is the loss of muscle mass that occurs with aging. The progressive loss of muscle mass in elderly people may contribute to falls and frailty in older adults.
What are some lifestyle changes that will extend your lifespan?
The most important lifestyle changes that can extend your lifespan are stopping smoking, reducing alcohol consumption, limiting saturated fats, maintaining a healthy weight and exercising regularly. Individuals who live a healthy lifestyle tend to enjoy better health and quality of life as they age.
What is ageism?
Ageism refers to a deep-seated belief that the young are capable and independent, while the elderly are incapable and dependent. Ageism can lead to discrimination against elderly individuals in a number of settings including education, housing, employment, and interpersonal relationships.
What is the difference between a cold and flu?
A cold is a viral infection caused by one of more than 100 different viruses. The usual symptoms of a cold are stuffy nose, watery eyes, sneezing, and cough. The flu (or “influenza”) is also viral, but it is usually more severe. Symptoms include high fever, fatigue, and general aches. Not everyone who has the flu develops serious complications, but for some people it can be fatal. The flu can also make existing medical conditions worse or trigger conditions like pneumonia or congestive heart failure when they are not present in healthy individuals.
Some bacterial infections can cause the symptoms of the flu, and they spread quickly in places where many people are living together. The symptoms are similar to those of the flu but can include chills, shaking and a general feeling of being unwell. If you experience these symptoms it is important to seek advice from your doctor as soon as possible.
What is influenza?
Influenza, commonly called ‘the flu’, is an infectious respiratory disease caused by the influenza virus. There are four groups of influenza virus, of which two (influenza A and B) are known to cause seasonal influenza epidemics in humans.
Symptoms of influenza often include fever, runny nose, sore throat, muscle pain, headache, coughing, and fatigue. Influenza infections may lead to pneumonia or other complications that can potentially be dangerous or fatal, especially in infants, the elderly, and those with existing health problems such as asthma or heart disease.
How is influenza spread?
Influenza is thought to be transmitted primarily through respiratory droplets produced when coughing or sneezing. The virus can also be transmitted through aerosols and on contaminated objects.
It is widely thought that influenza is highly infectious and mainly spread by sick people, however, not all evidence is consistent with this view. For example, it is argued that influenza is not infectious enough to account for the abrupt and explosive nature of epidemics.
One theory is that influenza is passed around by a subpopulation of people who are, for reasons unknown, particularly good at transmitting the virus. These individuals may generally be asymptomatic outside of the flu season, due to enhanced immunity as a result of sunlight exposure (and hence vitamin D production). This would mean that influenza would already be widely seeded in the population when the flu season arrived.
How long does it take to develop symptoms of influenza after being exposed?
The incubation period of influenza varies from one to four days.
Can influenza be spread asymptomatically?
Yes – a person may be infectious from 1 day before symptoms start until 5–7 days after illness onset. It is also hypothesised that some people may spread influenza without ever becoming ill outside of the flu season.
Why is influenza seasonal?
While flu cases still occur throughout the year, influenza epidemics usually occur during the colder months in temperate regions. Multiple factors may contribute to this, including:
- People spending more time indoors, creating more opportunities for the virus to spread.
- Lack of sunlight, which results in reduced vitamin D production leading to compromised immune function.
- The influenza virus itself may survive better in colder, drier conditions.
- Clearance of mucous (and the pathogens trapped within) from the airways may be impaired by cold conditions.
Where does the virus go in between epidemics?
Influenza is still able to spread outside of the flu season – just not as effectively. Non-seasonal outbreaks may still occur in more susceptible groups, such as in nursing homes. It has also been hypothesised that influenza is passed around outside the flu season by a subpopulation of asymptomatic people who are particularly good at transmitting the virus. This would mean that influenza would already be widely seeded in the population when the flu season arrived.
Why do we need a new influenza vaccine every year?
Influenza viruses mutate frequently, resulting in small changes to the structure of their antigens – the surface molecules that are recognised by the immune system. While most of these mutations do not significantly alter the viral antigens, they accumulate over time – this is called antigenic drift. In this way, viruses diverge until the previous vaccines no longer provide adequate protection against infection.
Each year, countries collect data about which influenza viruses are making people sick, the extent to which those viruses are spreading, and how well the previous season’s vaccine protects against those viruses. Vaccine composition is then updated accordingly.
Does getting vaccinated mean you can’t catch the flu?
No – unfortunately, the level of protection provided by influenza vaccines varies and is not 100%. Influenza vaccines tend to work best in healthy adults and older children, though they are still effective in preventing hospitalisation of individuals who contract the disease despite having been vaccinated.
Furthermore, there are many different influenza viruses in circulation at once, not all of which will be included in the seasonal flu vaccine. The vaccine is designed to immunise against the viruses that research suggests will be most common.
Finally, vaccines do not work instantly, and so it is possible to catch the virus just before or soon after vaccination – in this case the vaccine may not prevent you from becoming ill.
What causes influenza pandemics like the Spanish flu in 1918?
Influenza pandemics are rare, and only influenza A viruses are known to cause pandemics. They can occur when mutation results in a significant and abrupt change in the influenza A virus’s antigens – this is called antigenic shift. This can also occur when an influenza A virus from the animal population gains the ability to infect humans. Antigenic shift may produce antigens so different from those of currently circulating viruses that most people do not have any immunity against them.
How does influenza actually kill people?
Most flu deaths are caused by the body’s own immune system reacting either to the influenza virus or to a secondary infection, usually from bacteria. The immune system operates in a delicate balance: the immune response must be aggressive enough to eliminate pathogens, while limiting collateral damage to the body’s own tissues. In most healthy adults, this balance is correct and they recover within a few weeks at most. In others, inflammation may damage the lungs, cause them to fill with fluid, and impair oxygen exchange. A wide range of other complications are possible, such as septic shock (a widespread inflammatory response which causes a life-threatening drop in blood pressure).
What is the best way to protect yourself against influenza?
The most effective way to avoid catching influenza is to take the seasonal influenza vaccine. Limiting contact with sick people and maintaining good hygiene will reduce the risk of catching and transmitting the disease.
Can taking vitamin D supplements prevent influenza?
Vitamin D supplements will not prevent you from catching influenza, but may offer some level of protection depending on your unsupplemented vitamin D levels. Vitamin D is used for the production of antimicrobial peptides by the immune system, and there is evidence that supplementation may protect against respiratory infections including influenza, especially in those with lower vitamin D levels.
What is Toxoplasma gondii?
Toxoplasma gondii is a single celled parasite that infects virtually all warm-blooded animals including humans. It is estimated that between 30% and 50% of the world’s human population may be chronically infected. Toxoplasma rarely causes severe disease in humans, though it can be fatal to those with compromised immune systems, and also has a small risk of causing miscarriage if contracted during pregnancy. Following the initial infection, Toxoplasma evades destruction by the immune system by forming tiny cysts throughout the body. This state is permanent, but causes no visible symptoms. In this state, reinfection is not possible. For more information on Toxoplasma gondii, check out this article.

A cyst containing T. gondii in the brain of a mouse
Image source
How is Toxoplasma gondii spread?
The definitive hosts for Toxoplasma gondii are felines, which spread the parasite in their faeces during the initial stage of infection. Other animals may be infected by consuming food or water that is contaminated by cat faeces. Consuming the meat of an animal infected by Toxoplasma may also result in infection. This is likely to be the most common cause of Toxoplasma infection in humans, and can be prevented by ensuring that meat is properly cooked. This article contains a more detailed explanation of the life cycle of Toxoplasma.
Can Toxoplasma gondii affect human behaviour?
Some studies have shown Toxoplasma gondii to be associated with behavioural changes in humans, such as slower reaction times, increased aggression, and even with mental illness (schizophrenia). However, it is not known whether Toxoplasma causes these changes, or whether they are simply correlated with increased exposure to infection. This article explores the behaviour-modifying effects of Toxoplasma in more detail.
Copyright © Gowing Life Limited, 2024 • All rights reserved • Registered in England & Wales No. 11774353 • Registered office: Ivy Business Centre, Crown Street, Manchester, M35 9BG.