What Is Stem Cell Exhaustion? – The Hallmarks Of Ageing Series
Posted on 8 June 2021

|
Getting your Trinity Audio player ready...
|
In this series of articles, we discuss the nine ‘common denominators’ of the ageing process – the hallmarks of ageing. What exactly they are, how they change during ageing, and how we might be able reverse them in the future? Hopefully, by the end of this series, you will have a wider understanding of what actually makes us age.

Source
What Is Stem Cell Exhaustion?
At the moment of fertilisation, a cellular program launches to grow a new multicellular organism. This process, taking place before foetal development, is called embryogenesis and begins with a fertilised egg – a zygote. The zygote contains the 23 human chromosome pairs necessary to create a new person, one set from each parent. This zygote then undergoes multiple rounds of division to create a ball of cells called a blastocyst. Within this ball lies a cell mass consisting of embryonic stem cells, each with the ability to differentiate into one of many cell types within our bodies – this is called pluripotency. As we grow, the vast majority of our cells lose this ability to differentiate, but some pockets of cells do retain the ability to give rise to a limited set of cell types. This limited capacity to differentiate is called multipotency, and it helps us to renew and repair tissues across our body.
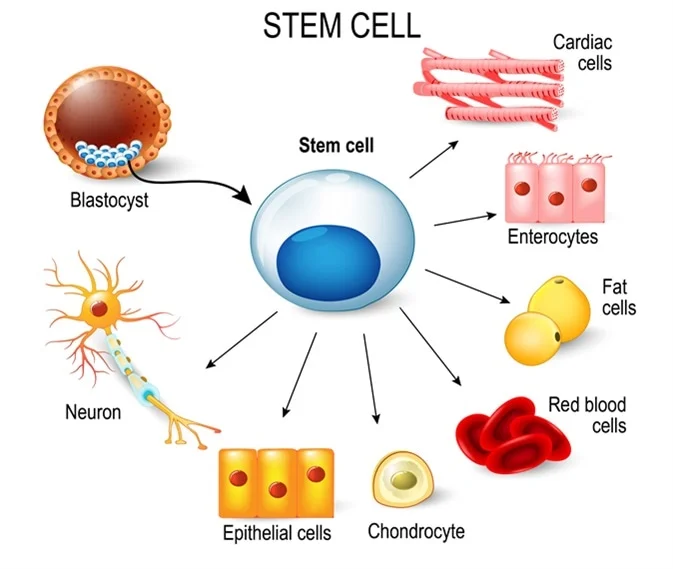
Source
The bulk of our cells are differentiated and actually don’t divide, instead being constantly replaced by a minority of proliferating cells. Haematopoietic stem cells in our bone marrow produce a constant supply of red and white blood cells, muscle stem cells stand at the ready to divide and repair damaged muscle tissue when needed, and dead skin cells are replaced on a daily basis by stem cells at the base of the epidermis. The nervous system and heart can be partly renewed but undergo minimal specific renewal in comparison to the rest of the body; new neurons can only form in certain areas. Stem cells are key to both providing new cells as old ones are lost and to correcting incurred wear and tear. Stem cell exhaustion refers to a decline in stem cell numbers and renewal capacity. Without stable populations of proliferating stem cells, tissues and organs lose their ability to recover from damage and begin to fail.

Source
An introduction to stem cell exhaustion by the American Aging Association
How Are Ageing And Stem Cell Exhaustion Linked?
Ageing is accompanied by a clear decline in regenerative capacity. Stem cell numbers in youth are higher, and injuries or illnesses that we can recover from quickly when we’re young can incapacitate us in our later years. The ability of our immune systems to fight off pathogens is tied to our ability to produce a large number of white blood cells in response. As relevant stem cells become exhausted, we struggle to maintain or create an effective army and infections become harder to fight.
The depletion of stem cells is caused in part by the shortening of telomeres (see telomere attrition). Multipotent stem cells are protected to some extent by higher levels of telomerase, which you may recall is the enzyme that prevents telomere shortening. However, these cells still cannot divide indefinitely, and will eventually begin to die or enter senescence as a result of telomere attrition or the accumulation of damage as discussed in previous articles. In most tissues, however, a larger part of stem cell ageing can be attributed to epigenetic changes (see epigenetic alterations) which activate or silence particular genes and prevent cells from doing their job. Genomic instability and epigenetic changes are passed on to daughter cells during division, leading to increased numbers of defective stem cells.

Source
Stem cell exhaustion seems to play a central role in various specific diseases of ageing such as sarcopenia, but how important are stem cells in organismal ageing as a whole? Research suggests that transplantation of muscle stem cells from young mice to mice that age prematurely can improve their lifespan, and also reduces degenerative changes in tissues besides those receiving new stem cells. This suggests that healthy stem cell populations aren’t just important for the tissues in which they reside, but also benefit the organism as a whole through factors they secrete.
How Can Stem Cell Exhaustion Be Measured?
The function of a population of stem cells can be assessed by measuring markers associated with stem cell exhaustion, including apoptosis, senescence, gene expression, and certain signalling pathways. For example, the activity of the Notch and Wnt signalling pathways can serve as markers for stem cell proliferation and self-renewal. The ability of stem cells to proliferate can also be measured directly in cell culture.
Unfortunately, it is difficult to assess stem cell function non-invasively in humans – at least until the clinical consequences of stem cell exhaustion (such as sarcopenia and loss of immune function) manifest. One potential marker of stem cell function that has recently attracted interest is the level of circulating osteogenic progenitor cells. These are stem cells that circulate in the blood and are able to differentiate into various tissue types including bone and muscle. Their levels in the circulation may serve as a marker for the health of certain stem cell populations.
How Might Stem Cell Exhaustion Be Fixed?
It should in theory be possible to slow or reverse some aspects of the ageing process by replenishing and replacing damaged stem cells. However, this requires a safe and relatively easy means of producing new stem cells, which has historically been a major challenge. Since Shinya Yamanaka succeeded in generating pluripotent stem cells from fully differentiated cells in 2006, we have made significant progress in this regard. It is now possible to take differentiated cells from a patient, reprogram those cells into stem cells, and reintroduce those cells into tissues to treat disease. This reprogramming not only changes a differentiated cell into a stem cell, but can also ‘reset’ that cell to a younger state. We already knew this resetting must be possible, since stem cell age is reset in an organism’s offspring, regardless of parental age. It is now becoming possible to initiate this reprogramming in cells of our choosing.
In 2020, there were over 3000 clinical trials involving the use of adult stem cells in the WHO International Clinical Trials Registry. Due to the risk of genetic and epigenetic alterations taking place during the induction of stem cells, and the risk of tumour formation as a result of stem cell transplantation, human trials of any stem cell therapy must be approached with caution. Some clinical trials are tentatively investigating the general anti-ageing potential of stem cell infusion. Unfortunately, a growing number of private clinics are already selling unproven (and potentially unsafe) stem cell therapies to treat ageing and a variety of ailments. Some of these interventions might work, but there is currently insufficient clinical evidence to support their effectiveness and their safety, with severe consequences for some recipients.
What about preventing or reversing stem cell exhaustion itself, rather than simply replacing defective stem cells? As discussed earlier, most stem cell exhaustion is the result of two other hallmarks of ageing: epigenetic alterations and telomere attrition. Through treatment of these hallmarks, we might expect to see attenuation of stem cell exhaustion. Some general examples of how this might be achieved are discussed in previous articles of this series. Various genes have been identified as being particularly important in stem cell ageing, and could potentially be targeted therapeutically. For example, KL is an anti-ageing gene encoding the Klotho protein, which acts through a variety of mechanisms. Overexpression of Klotho in animals results in increased lifespan, while deficiency promotes telomere shortening and stem cell exhaustion. As touched upon earlier, stem cell function appears to be linked to systemic signals and effects throughout the body, and some evidence suggests that factors in the blood of young organisms (of which klotho may be one) can have beneficial effects on stem cells in older organisms.
References
Current state of stem cell-based therapies: an overview: https://dx.doi.org/10.21037%2Fsci-2020-001
Tumorigenicity as a Clinical Hurdle for Pluripotent Stem Cell Therapies: https://dx.doi.org/10.1038%2Fnm.3267
MSC Infusion for Anti-aging and Regenerative Therapy (REGEN) NCT04174898: https://clinicaltrials.gov/ct2/show/NCT04174898
Untested, unproven, and unethical: the promotion and provision of autologous stem cell therapies in Australia: https://dx.doi.org/10.1186%2Fscrt543
Klotho, stem cells, and aging: https://dx.doi.org/10.2147%2FCIA.S84978
Klotho Deficiency Accelerates Stem Cells Aging by Impairing Telomerase Activity: https://dx.doi.org/10.1093%2Fgerona%2Fgly261
The central role of muscle stem cells in regenerative failure with aging: https://dx.doi.org/10.1038%2Fnm.3918
Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model: https://doi.org/10.1038/ncomms1611
Effect of aging on stem cells: https://dx.doi.org/10.5493%2Fwjem.v7.i1.1
Rejuvenation of aged progenitor cells by exposure to a young systemic environment: https://doi.org/10.1038/nature03260
Copyright © Gowing Life Limited, 2024 • All rights reserved • Registered in England & Wales No. 11774353 • Registered office: Ivy Business Centre, Crown Street, Manchester, M35 9BG.



You must be logged in to post a comment.