What Is Telomere Attrition? – The Hallmarks Of Ageing Series
Posted on 21 May 2021

|
Getting your Trinity Audio player ready...
|
In this series of articles, we discuss the nine ‘common denominators’ of the ageing process – the hallmarks of ageing. What exactly they are, how they change during ageing, and how we might be able reverse them in the future? Hopefully, by the end of this series, you will have a wider understanding of what actually makes us age.

Source
What Is Telomere Attrition?
A human being’s body experiences about 10,000 trillion cell divisions in a lifetime. Successful cell division is absolutely crucial for life, facilitating repair and growth. The most important part of every single division is the precise replication of the cell’s DNA. Each time a cell divides, it must make a near perfect copy of its genetic information to pass on to its daughter cells. However, the molecular machinery that carries out this copying process has a problem – it cannot quite replicate the entire length of the DNA strand, meaning that a portion of the DNA at the end of each chromosome is lost each time the cell divides.
This is where telomeres come into the story. Telomeres are sequences of repetitive DNA that serve to protectively cap the ends of the chromosomes. Like the rest of a chromosome, including its genes, telomeres are stretches of chemical DNA code. However, the telomeric regions contain no actual useful genetic information and can be likened to the plastic tips on shoelaces, protecting the ends of the chromosomes from damage. When a cell divides and its DNA is copied, a section of the DNA is lost from the ends of the telomeric regions instead of the regions containing genes essential for life. This is called telomere attrition or telomere shortening. Thus, telomeres allow cells to divide without losing crucial genetic information.

Source
In addition to preserving important genetic code during cell division, telomeres act like the markers of the end of DNA strands, allowing the cell to recognise them as such. Without telomeres, the ends of different chromosomes could fuse together, dramatically altering the cell’s genetic makeup in a way that would lead to malfunction, cancer, or cell death. Aptly named shelterin proteins are associated with the telomeres to distinguish them from breaks in the DNA. Without these proteins, a cell would recognise the DNA ends as chromosome damage and try to fix something that was not broken. This would also cause the cell to stop dividing and eventually die. Therefore, telomeres protect against both chromosome deterioration and fusion with neighbouring chromosomal ends.
An introduction to telomere attrition by the American Aging Association.
How Does Telomere Attrition Relate To Ageing?
Telomeres are only able to protect the chromosomes for so long. Every time the cell divides and DNA replicates, the telomeres will become shorter and shorter. In some cells, an enzyme called telomerase is able to repair these telomeres, but the majority of normal mammalian cells do not express telomerase, meaning that telomeres will continue to shorten until further replication risks eating into the genetic code. To prevent this from happening, cells will usually stop dividing when their telomeres become too short (this is called replicative senescence) or ‘commit suicide’ (apoptosis). This replication limit is called the Hayflick limit and occurs in most human cells after around 40 – 60 divisions, at least in cells cultured in the lab. It is still a matter of debate whether the Hayflick limit is as important in living organisms as it is in cell culture, since these two environments are very different and have substantial effects on how cells behave.

Source
Telomere exhaustion is observed in ageing and represents a type of molecular clock that drives the ageing process, progressively reducing the ability of tissues to repair and regenerate themselves. Meta analyses indicate that shortened telomeres are correlated with increased mortality risk in humans, suggesting that telomere length may be a factor in lifespan, though questions remain about just how important it is. Mice genetically modified to have longer or shorter telomeres have longer or shorter lives, respectively. However, mouse cells have longer telomeres than human cells, yet have a smaller Hayflick limit and become senescent faster, so telomere length is not the only important factor. In both humans and animals, reduced expression of the telomere repair enzyme telomerase correlates with increased disease incidence, while genetically enhancing the expression of telomerase makes mice live longer. This hints that the rate at which telomeres are degraded or repaired may be more important than their initial length when it comes to an organism’s lifespan.
How Can Telomere Attrition Be Measured?
Measuring telomere length first requires a DNA sample from the cells to be studied. The first technique used to measure telomere length involved degrading all of the non-chromosomal DNA, using enzymes that attacked sites not found within telomeres. The length of the pieces of DNA that remained (the telomeres) can then be measured using a technique called gel electrophoresis. The samples to be measured are put into wells within an agarose gel, and an electric current is applied. Since DNA is a negatively charged molecule, it will move through the gel. Smaller fragments will move further, and in this way the length of the telomeres can be measured.
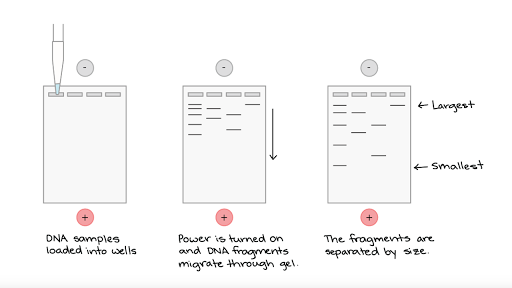
Source
Alternatively, telomere length can be measured using qPCR (quantitative polymerase chain reaction), a technique used to amplify and measure DNA in the lab. Molecules called primers are used to target a specific sequences within the DNA, which are then copied during each ‘cycle’ of qPCR. To measure telomere length, qPCR is used to copy DNA regions found within the telomeres, and the quantity of genetic material produced at each cycle is measured using a fluorescent probe. This is compared to the same measurements for a known gene that goes through the same number of qPCR cycles. The ratio between these two measurements allows the length of the telomeres to be estimated.

Source
Telomere length can also be measured using quantitative fluorescence in situ hybridization (Q-FISH), in which fluorescent probes are used to directly label telomeres within prepared cells.

Source
How Might Telomere Attrition Be Fixed?
Given the evidence that people with longer telomeres do live longer and that animal lifespan can be extended through telomere restoration, fixing telomere attrition could be a major pathway for preserving tissue function and essentially slowing the ageing process. However, we do need to be cautious, as telomere shortening does play a role outside of protecting the DNA, and that is protection against cancer. This is because if a cell mutates and begins to divide uncontrollably, its telomeres will rapidly become too short to allow for further division. This is why in order to become cancerous, cells must acquire mutations that either preserve their telomeres, or that break this failsafe system entirely and allow them to escape replicative senescence even when their telomeres become short.

Source
Sure enough, mice that are modified to express telomerase (the enzyme that repairs telomeres) throughout life have slightly increased rates of cancer in return for a reduced rate of ageing. However, interestingly, reactivating telomerase in adult mice slows ageing and increases lifespan, seemingly without increasing cancer incidence. It may therefore be possible to derive benefits from telomere repair without the downsides. There exist chemicals such as TA-65, extracted from the Astragalus Membranaceus plant, that can activate telomerase in humans, and which has been reported to improve aspects of health in human volunteers. Telomerase activation might therefore be the most likely path to fixing telomere attrition, though many questions about the exact relationship between telomeres and ageing remain to be answered, and much more research is needed before we are all taking telomerase activators.
References
The Hallmarks of Aging: https://dx.doi.org/10.1016%2Fj.cell.2013.05.039
Telomerase reverse transcriptase delays aging in cancer-resistant mice: https://doi.org/10.1016/j.cell.2008.09.034
Short telomeres are sufficient to cause the degenerative defects associated with aging: https://doi.org/10.1016/j.ajhg.2009.10.028
Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer: https://doi.org/10.1002/emmm.201200245
Potential of telomerase activation in extending health span and longevity: https://dx.doi.org/10.1016%2Fj.ceb.2012.09.004
Discovery of potent telomerase activators: Unfolding new therapeutic and anti-aging perspectives: https://dx.doi.org/10.3892%2Fmmr.2019.10614
Telomerase activation: A potential key modulator for human healthspan and longevity: https://doi.org/10.1016/j.arr.2013.12.006
Telomere length behaves as biomarker of somatic redundancy rather than biological age: https://doi.org/10.1111/acel.12050
Copyright © Gowing Life Limited, 2024 • All rights reserved • Registered in England & Wales No. 11774353 • Registered office: Ivy Business Centre, Crown Street, Manchester, M35 9BG.

