
|
Getting your Trinity Audio player ready...
|
In 1990, a 4-year-old girl named Ashanthi DeSilva received a new treatment for severe combined immunodeficiency, a genetic disease resulting in high susceptibility to severe and often fatal infections. DeSilva’s condition was caused by the lack of a key enzyme called adenosine deaminase (ADA), which leads to the inhibition of T cell and B cell division. The new 12-day treatment involved using a virus to introduce a healthy gene encoding the ADA enzyme into DeSilva’s immune cells. The treatment was successful, improving her immune function enough for her to live a relatively normal life. This was the first approved use of gene therapy in humans and a landmark scientific achievement.

Broadly speaking, gene therapy is the modification or manipulation of gene expression with the aim of curing disease. This includes inactivating or replacing mutated genes, as well as the introduction of new genes to treat disease. It has immense potential to improve health and longevity not only in people with genetic diseases like in Ashanthi DeSilva’s case 30 years ago, but also in those with non-genetic diseases such as cancer with the advent of CAR-T cell therapy.

BioProcess International
While gene therapy technology holds great promise, it has had a troubled past. Clinical trials in the 1990s revealed serious dangers such as severe inflammatory responses to the vector (the means of getting the gene therapy into the target cells) as well as the activation of cancer-associated genes. The science around the technique was revisited, which lead to the development of safer gene therapies in the 2000s. These safer techniques were able to demonstrate clinical benefits for patients in early trials, however, it still took until 2017 for the U.S Food and Drug Administration to approve the first gene therapy products. Meanwhile Glybera, the first gene therapy to be approved in the European Union, was removed from the market in 2017 due to being unprofitable.
Since then, the field of gene therapy seems to have shifted gears. The pace of development and approval of new gene therapies has accelerated. There are currently hundreds of ongoing clinical trials for gene therapies, and the FDA estimated it would be approving 10-20 gene therapies a year come 2025. Now, we are on the cusp of seeing unprecedented application of gene therapy in the form of the Oxford/AstraZeneca adenovirus vaccine. While they don’t alter the recipient’s genes or gene expression, adenovirus vaccines are still technically a branch of gene therapy: a modified adenovirus is used to introduce a new gene (in this case encoding the Sars-CoV-2 spike protein) into cells to induce immunity.

The New York Times
After decades of setbacks, it appears that gene therapy has finally ‘arrived’. So, what lead this to happen now? It’s difficult to say for sure, but it seems to be the result of multiple factors coming together over the last few years.
Gene Therapy Innovations
Technological advances in the last decade have greatly expanded the scope of what gene therapies can be used to do while also improving their effectiveness. Development of better viral vectors for delivering genes to diseased cells plays a considerable role here. Of particular note are advances in so-called adeno-associated viruses (AAVs), which don’t cause disease in humans, can be targeted to specific cell types, and can elicit gene expression for up to 10 years.
Another key player is CRISPR gene editing, a technique that can be used to cut out and swap in sections of DNA, or even alter a single letter of the genetic code. This allows not only for healthy genes to be introduced, but crucially also allows for the diseased gene to be removed. CRISPR technology has progressed rapidly to clinical trials, and continues to be refined and expanded upon.

The Royal Swedish Academy of Sciences – Johan Jarnestad
We have also seen developments in approaches acting at the level of gene expression rather than at the level of genes themselves. RNA interference – the introduction of certain types of RNA – has now been used successfully to ‘silence’ mutated genes in humans to treat disease.
In summary, the breadth of gene therapy has continuously been expanding beyond simply ‘replacing bad genes with good genes’, and we are now seeing the fruits of these innovations.
Safety and Regulation
Safety has been a major concern in the field of gene therapy since its inception, resulting in tighter control by regulatory authorities. By its nature, gene therapy has long lasting consequences that are challenging to reverse. Techniques that involve making changes to the genetic code risk off-target action – that is to say, they may alter the sequence of genes besides the target gene, which could lead to the development of cancer. A bigger problem, at least in the earlier days of gene therapy, was the risk of severe immune reactions against the treatment. This is what lead to the death of Jesse Gelsinger in 1999.
Innovations in the field of gene therapy since then have largely eliminated these concerns. This lead to the 2018 announcement that gene therapies no longer required review by US National Institutes of Health (NIH) advisory committee on recombinant DNA. The NIH director wrote:
There is no longer sufficient evidence to claim that the risks of gene therapy are entirely unique and unpredictable – or that the field still requires special oversight that falls outside our existing framework for ensuring safety.
In other words, gene therapy is being viewed less and less as a ‘special case’ that requires additional oversight when it comes to safety, and this has surely allowed research and clinical trials to progress more rapidly.
A Word On Genome Sequencing
It may also be worth considering the impact that advances in genome sequencing – the reading of the complete DNA sequence of an organism’s genome – have on the potential applications of gene therapy. The development of faster and less costly genome sequencing technology means that sequencing an individual’s genome and giving them a personalised gene therapy is now a possibility.
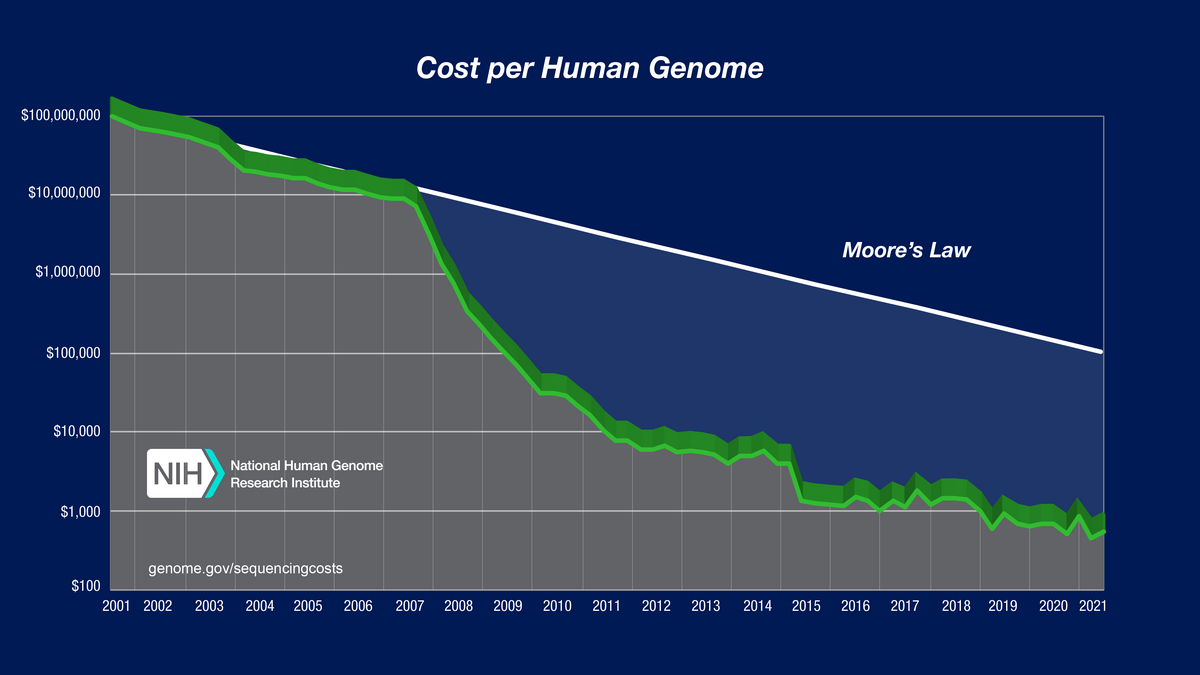
Modern sequencing has allowed us to amass large databases of human genetic information and identify mutations associated with common and complex diseases. Just as getting your genome sequenced may help you make better health and lifestyle decisions, it may one day form the basis of personalised gene therapy to permanently mitigate a genetic risk factor. Thus, genome sequencing greatly expands the group of people who may directly benefit from gene therapy. This is not something we are likely to see for some time, but possibilities like this may help drive gene therapy research forward today.
References
Adeno-associated virus vector as a platform for gene therapy delivery: https://doi.org/10.1038/s41573-019-0012-9
Gene editing and CRISPR in the clinic: current and future perspectives: https://dx.doi.org/10.1042%2FBSR20200127
The first human trial of CRISPR-based cell therapy clears safety concerns as new treatment for late-stage lung cancer: https://www.nature.com/articles/s41392-020-00283-8
History of gene therapy: https://doi.org/10.1016/j.gene.2013.03.137
Gene therapy comes of age: https://science.sciencemag.org/content/359/6372/eaan4672.long
The Return of Gene Therapy: https://www.labiotech.eu/in-depth/gene-therapy-history/
Regulating the gene-therapy revolution: https://www.nature.com/articles/d41586-018-07641-1
Copyright © Gowing Life Limited, 2024 • All rights reserved • Registered in England & Wales No. 11774353 • Registered office: Ivy Business Centre, Crown Street, Manchester, M35 9BG.


You must be logged in to post a comment.